BMRB Featured System: Dihydrofolate Reductase
| |||||
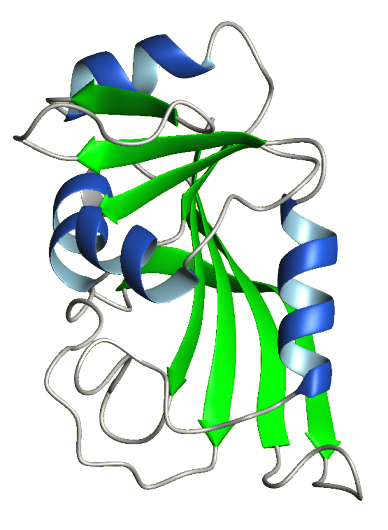
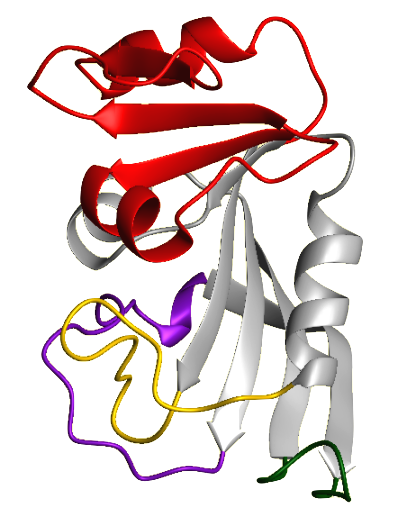
Dihydrofolate Reductase (DHFR; 5,6,7,8-tetrahydrofolate:NADP+ oxidoreductase) is a very important enzyme because it produces cofactors that are necessary for DNA synthesis. Specifically, DHFR catalyzes the reduction of folate and 7,8-dihydrofolate (DHF) to 5,6,7,8-tetrahydrofolate (THF). THF is an essential cofactor involved in the transfer of methyl, methylene, and formyl groups from one molecule to another during the production of nucleotides and several amino acids. An important example of this is the utilization of carbon units from a THF cofactor by thymidylate synthase to make thymidine nucleotides. DHFR is found ubiquitously in all dividing cells of prokaryotes and eukaryotes. The mammalian enzymes are all highly similar in sequence, while each bacterial form is distinct. The DHFR sequence in humans is 30% similar to that of E. coli and 70% similar to other mammalian DHFRs. The standard source for the enzyme is the mammalian and avian liver. Human DHFR is a 186 amino acid protein with a molecular weight around 20 kDa. Structural studies show that DHFR is a monomeric molecule with many secondary structural elements. These include an eight stranded beta-sheet and four alpha helices with connecting loop regions. The protein is divided into two subdomains, the adenosine-binding subdomain and the loop subdomain. The adenosine-binding subdomain is the larger of the two and binds the adenosine moiety of NADPH. The loop subdomain contains three loops, the Met-20 loop, the F-G loop, and the G-H loop. Between the two subdomains is the active site, a long groove where folate and NADPH bind. The size of the active site is regulated by the movements of the two subdomains. DHFR was discovered in the late 1950s by investigators searching for folate-dependent enzymes involved in 1-carbon metabolism. Because of DHFRs vital role in DNA synthesis, it was targeted for cancer chemotherapy. In the early 1950s, the drug methotrexate proved to be useful in the treatment of many kinds of cancers, so there was a demand to identify its intracellular target. Methotrexate mimics the folate molecule and competitively binds to the active site of DHFR, inhibiting it. In 1957, Sidney Futterman fractionated a chicken liver extract and obtained a preparation that catalyzed the reduction of both folate and DHF to THF. He also found that methotrexate and aminopterin, another anticancer drug, inhibited both of these processes. The following year, Zakrzewski and Nichol produced a chicken liver preparation with the same properties and named it 'folate hydrogenase'. Later that year, Osborn and Huennekens recognized that DHF was the preferable substrate for the enzyme, so they dubbed the enzyme, 'dihydrofolate reductase', the name we use today. Dihydrofolate Reductase inhibitors have also been used to combat malaria. For example, the drug Pyrimethamine has been used in combination with Sulphadoxine to fight against Plasmodium falciparum, the bacteria that causes malaria. Pyrimethamine is an effective inhibitor of the bacterial DHFR enzyme and Sulphadoxine blocks the action of an enzyme necessary for the synthesis of folate in the bacteria. Together they make it very difficult for the bacteria to reproduce. The use of Pyrimethamine has caused new drug resistant strains of Plasmodium falciparum to emerge which has reduced the drug's effectiveness. Due to the drugs lessened effectiveness, the development of new antimalarial drugs that will be effective against the current drug-resistant strains is currently underway. DHFR was chosen as the Protein Data Bank's Molecule of the Month for October of 2002. The site provides useful background material on DHFR as well as information about DHFR antagonists. | |||||
|
Next: DHFR Catalysis |