D-(+)-Maltose (C12H22O11)
BMRB entry bmse000017
Entry DOI: doi:10.13018/BMSE000017
Data source: Madison Metabolomics Consortium - Francisca Jofre, Mark E. Anderson, John L. Markley
NMR-STAR file:
bmse000017.strNMR-STAR
interactive viewerStructure file (mol/sdf):
bmse000017.molAll files for
bmse000017Time Domain Data:
bmse000017.zipSample and instrument details are given with the spectrum
Assigned chemical shifts
Set 1
Sample: multiple; see NMR-STAR file
Conditions: temperature: 298K, pH: 7.4
Spectrometer: multiple; see NMR-STAR file
| Atom ID | Author Nomenclature | Value | Ambiguity Code |
|---|---|---|---|
| C10 | C1 | 79.490 | 4 |
| C10 | C1 | 79.288 | 4 |
| C10 | C1 | 78.956 | 4 |
| C10 | C1 | 77.280 | 4 |
| C10 | C1 | 76.727 | 4 |
| C10 | C1 | 75.978 | 4 |
| C10 | C1 | 75.562 | 4 |
| C10 | C1 | 75.396 | 4 |
| C10 | C1 | 74.422 | 4 |
| C10 | C1 | 74.014 | 4 |
| C10 | C1 | 72.662 | 4 |
| C10 | C1 | 72.033 | 4 |
| C4 | C2 | 79.490 | 4 |
| C4 | C2 | 79.288 | 4 |
| C4 | C2 | 78.956 | 4 |
| C4 | C2 | 77.280 | 4 |
| C4 | C2 | 76.727 | 4 |
| C4 | C2 | 75.978 | 4 |
| C4 | C2 | 75.562 | 4 |
| C4 | C2 | 75.396 | 4 |
| C4 | C2 | 74.422 | 4 |
| C4 | C2 | 74.014 | 4 |
| C4 | C2 | 72.662 | 4 |
| C4 | C2 | 72.033 | 4 |
| C7 | C4 | 79.490 | 4 |
| C7 | C4 | 79.288 | 4 |
| C7 | C4 | 78.956 | 4 |
| C7 | C4 | 77.280 | 4 |
| C7 | C4 | 76.727 | 4 |
| C7 | C4 | 75.978 | 4 |
| C7 | C4 | 75.562 | 4 |
| C7 | C4 | 75.396 | 4 |
| C7 | C4 | 74.422 | 4 |
| C7 | C4 | 74.014 | 4 |
| C7 | C4 | 72.662 | 4 |
| C7 | C4 | 72.033 | 4 |
| C2 | C6 | 63.446 | 4 |
| C2 | C6 | 63.297 | 4 |
| C2 | C6 | 63.168 | 4 |
| C12 | C7 | 102.28 | 1 |
| C8 | C8 | 79.490 | 4 |
| C8 | C8 | 79.288 | 4 |
| C8 | C8 | 78.956 | 4 |
| C8 | C8 | 77.280 | 4 |
| C8 | C8 | 76.727 | 4 |
| C8 | C8 | 75.978 | 4 |
| C8 | C8 | 75.562 | 4 |
| C8 | C8 | 75.396 | 4 |
| C8 | C8 | 74.422 | 4 |
| C8 | C8 | 74.014 | 4 |
| C8 | C8 | 72.662 | 4 |
| C8 | C8 | 72.033 | 4 |
| C11 | C10 | 98.490 | 4 |
| C11 | C10 | 94.614 | 4 |
| C9 | C13 | 79.490 | 4 |
| C9 | C13 | 79.288 | 4 |
| C9 | C13 | 78.956 | 4 |
| C9 | C13 | 77.280 | 4 |
| C9 | C13 | 76.727 | 4 |
| C9 | C13 | 75.978 | 4 |
| C9 | C13 | 75.562 | 4 |
| C9 | C13 | 75.396 | 4 |
| C9 | C13 | 74.422 | 4 |
| C9 | C13 | 74.014 | 4 |
| C9 | C13 | 72.662 | 4 |
| C9 | C13 | 72.033 | 4 |
| C3 | C16 | 79.490 | 4 |
| C3 | C16 | 79.288 | 4 |
| C3 | C16 | 78.956 | 4 |
| C3 | C16 | 77.280 | 4 |
| C3 | C16 | 76.727 | 4 |
| C3 | C16 | 75.978 | 4 |
| C3 | C16 | 75.562 | 4 |
| C3 | C16 | 75.396 | 4 |
| C3 | C16 | 74.422 | 4 |
| C3 | C16 | 74.014 | 4 |
| C3 | C16 | 72.662 | 4 |
| C3 | C16 | 72.033 | 4 |
| C6 | C17 | 79.490 | 4 |
| C6 | C17 | 79.288 | 4 |
| C6 | C17 | 78.956 | 4 |
| C6 | C17 | 77.280 | 4 |
| C6 | C17 | 76.727 | 4 |
| C6 | C17 | 75.978 | 4 |
| C6 | C17 | 75.562 | 4 |
| C6 | C17 | 75.396 | 4 |
| C6 | C17 | 74.422 | 4 |
| C6 | C17 | 74.014 | 4 |
| C6 | C17 | 72.662 | 4 |
| C6 | C17 | 72.033 | 4 |
| C5 | C19 | 79.490 | 4 |
| C5 | C19 | 79.288 | 4 |
| C5 | C19 | 78.956 | 4 |
| C5 | C19 | 77.280 | 4 |
| C5 | C19 | 76.727 | 4 |
| C5 | C19 | 75.978 | 4 |
| C5 | C19 | 75.562 | 4 |
| C5 | C19 | 75.396 | 4 |
| C5 | C19 | 74.422 | 4 |
| C5 | C19 | 74.014 | 4 |
| C5 | C19 | 72.662 | 4 |
| C5 | C19 | 72.033 | 4 |
| C1 | C20 | 63.446 | 4 |
| C1 | C20 | 63.297 | 4 |
| C1 | C20 | 63.168 | 4 |
| H35 | H24 | 3.764 | 4 |
| H35 | H24 | 3.412 | 4 |
| H35 | H24 | 3.266 | 4 |
| H29 | H25 | 3.764 | 4 |
| H29 | H25 | 3.412 | 4 |
| H29 | H25 | 3.266 | 4 |
| H32 | H26 | 3.764 | 4 |
| H32 | H26 | 3.412 | 4 |
| H32 | H26 | 3.266 | 4 |
| H37 | H29 | 5.41 | 1 |
| H33 | H30 | 3.764 | 4 |
| H33 | H30 | 3.412 | 4 |
| H33 | H30 | 3.266 | 4 |
| H36 | H32 | 5.223 | 4 |
| H36 | H32 | 4.648 | 4 |
| H34 | H34 | 3.764 | 4 |
| H34 | H34 | 3.412 | 4 |
| H34 | H34 | 3.266 | 4 |
| H28 | H37 | 3.764 | 4 |
| H28 | H37 | 3.412 | 4 |
| H28 | H37 | 3.266 | 4 |
| H31 | H38 | 3.764 | 4 |
| H31 | H38 | 3.412 | 4 |
| H31 | H38 | 3.266 | 4 |
| H30 | H40 | 3.764 | 4 |
| H30 | H40 | 3.412 | 4 |
| H30 | H40 | 3.266 | 4 |
NMR experiments
1: 1D 1H, 0.5 mM
Sample: 0.5mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
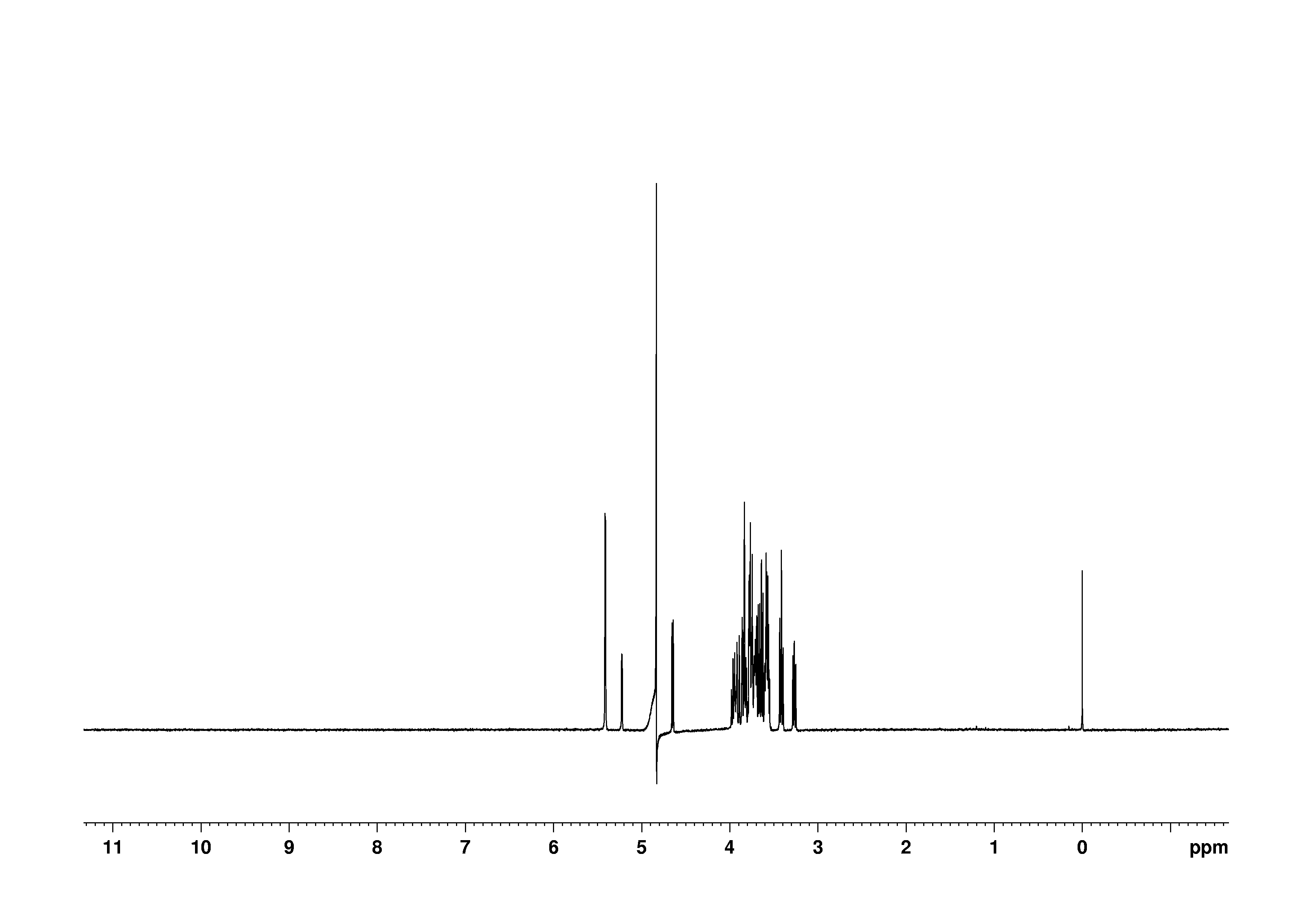
2: 1D 1H, 2.0 mM
Sample: 2.0mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
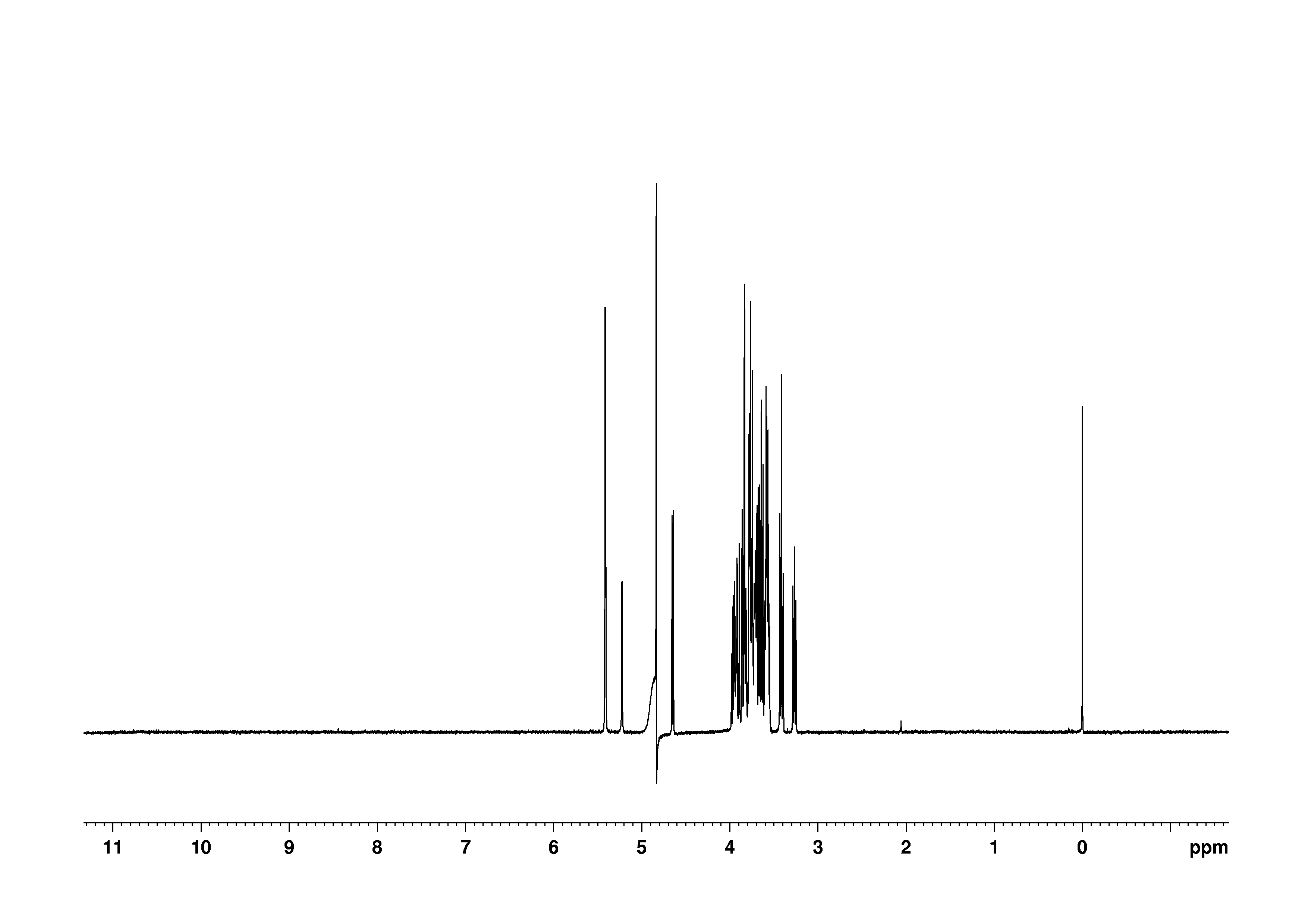
3: 1D 1H
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
4: 2D [1H,1H]-TOCSY
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #4: 2D [1H,1H]-TOCSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000017/nmr/set01/spectra/HH_TOCSY/00.png)
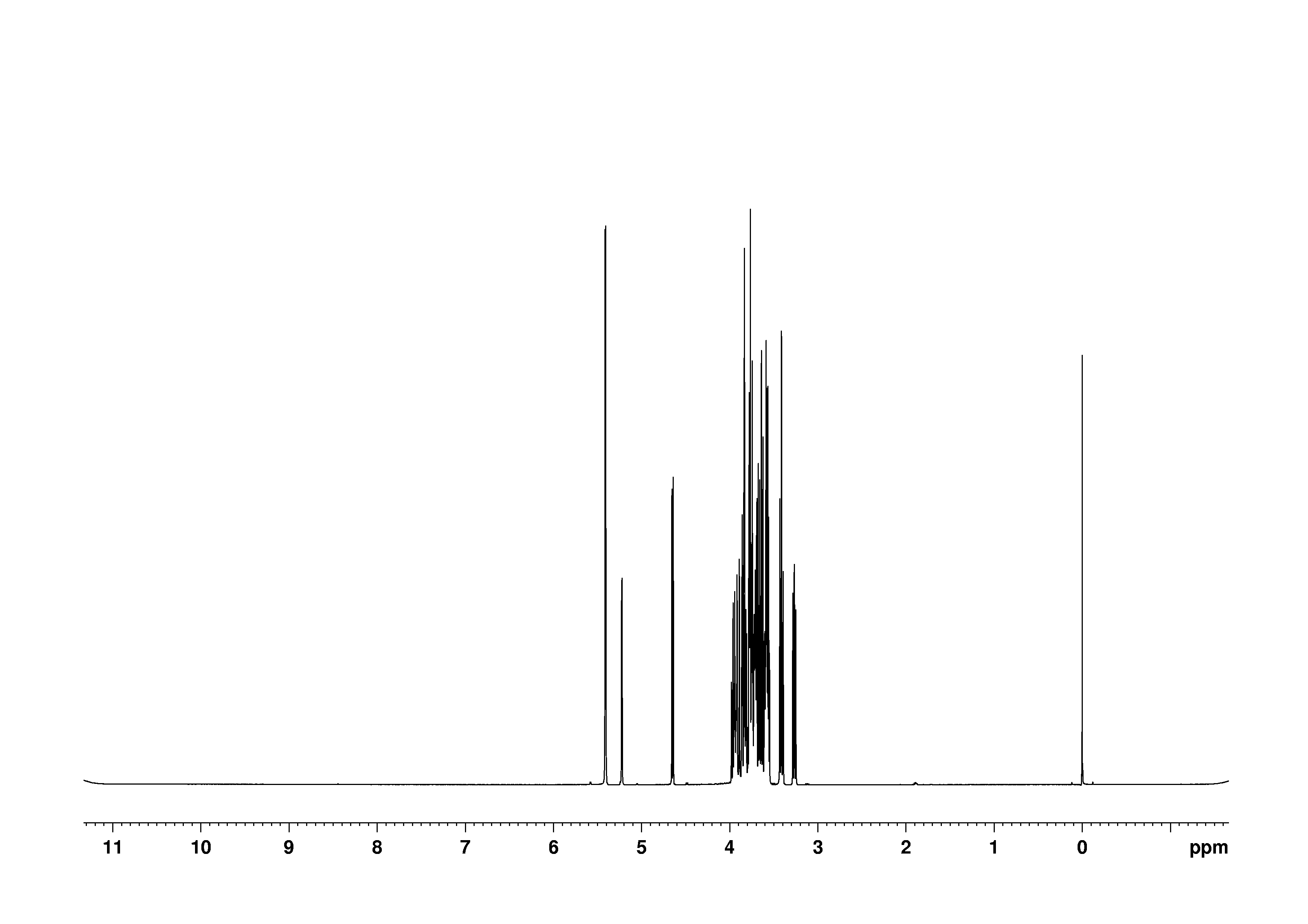
5: 1D 13C
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
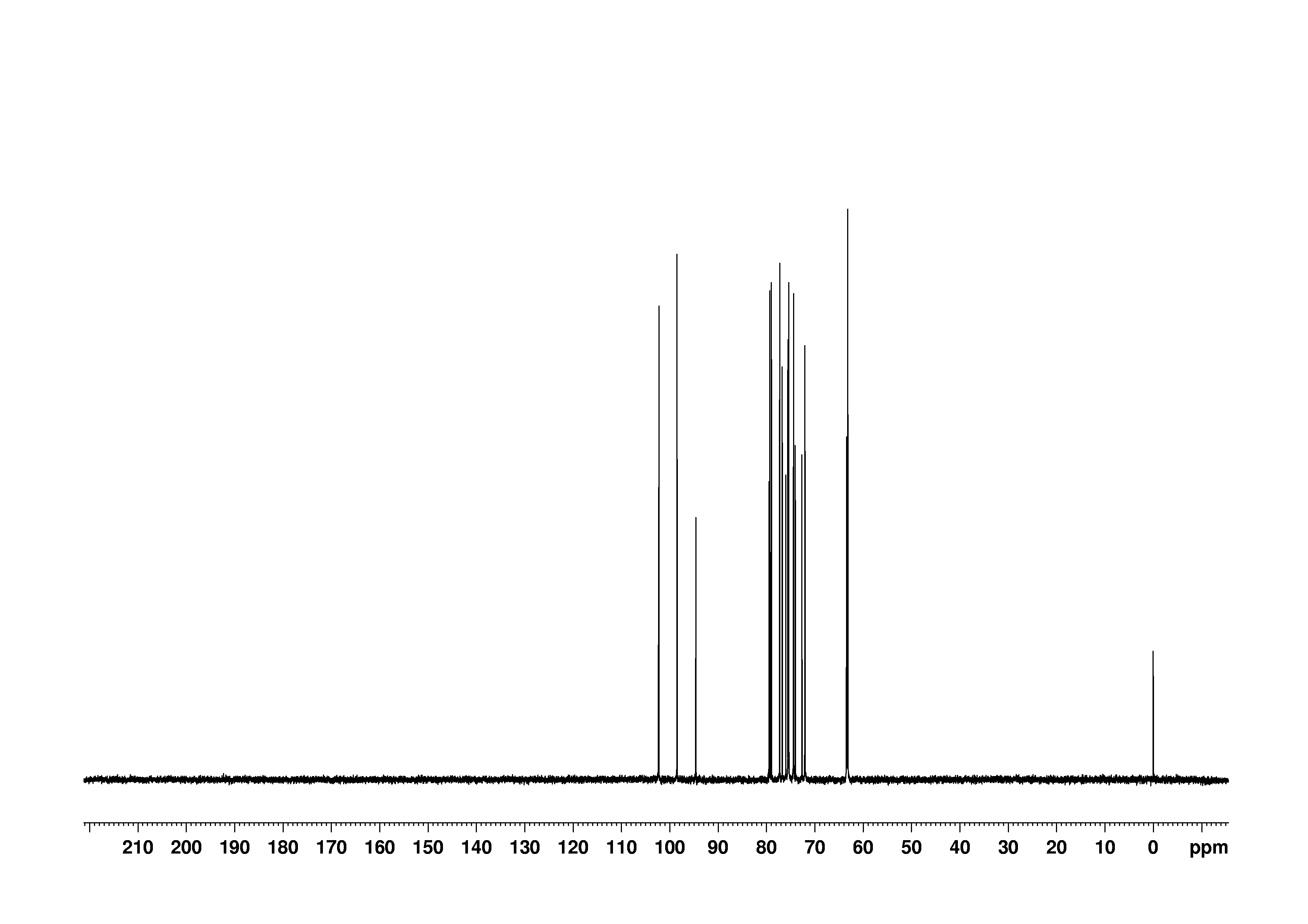
6: 1D DEPT90
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
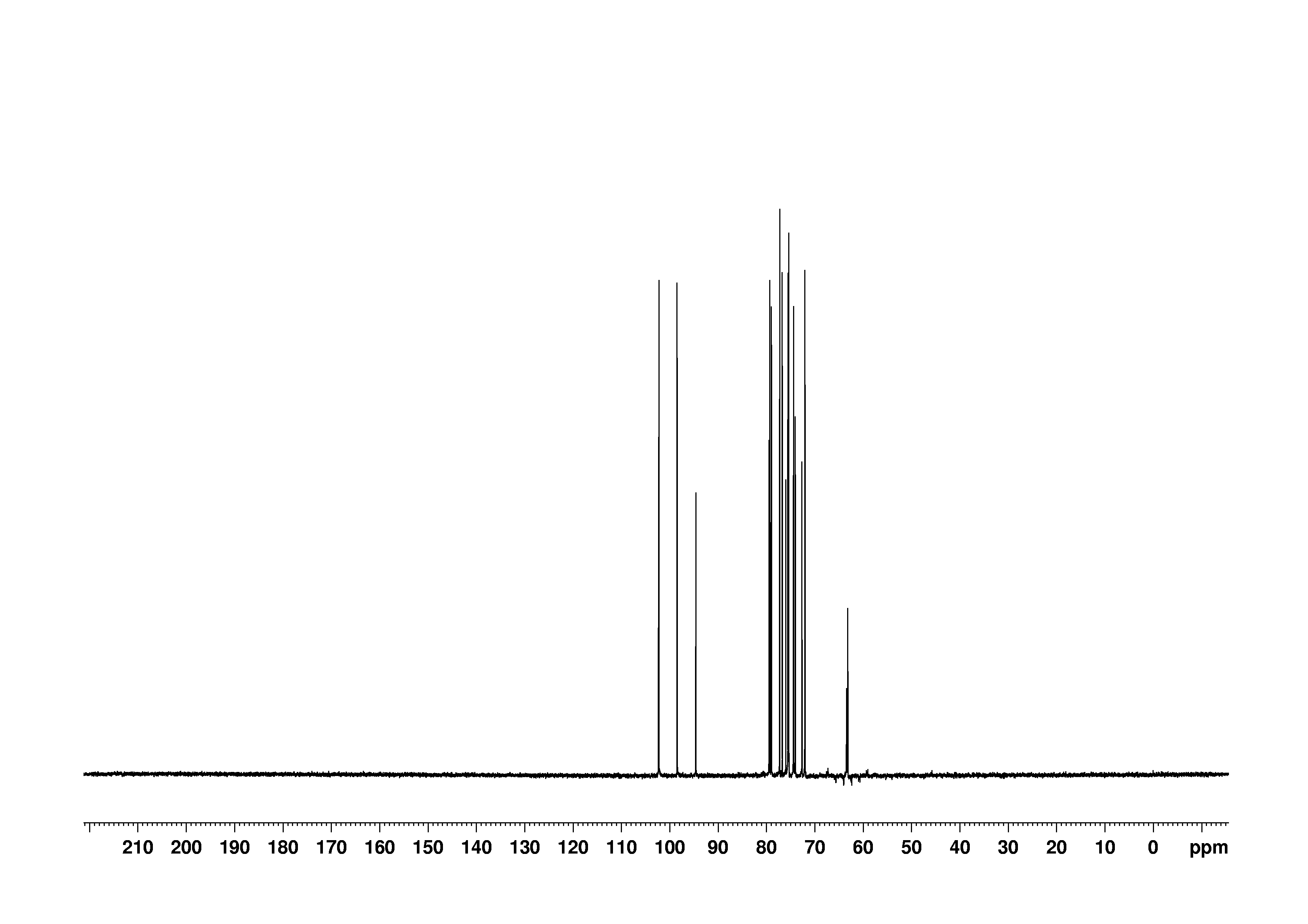
7: 1D DEPT135
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
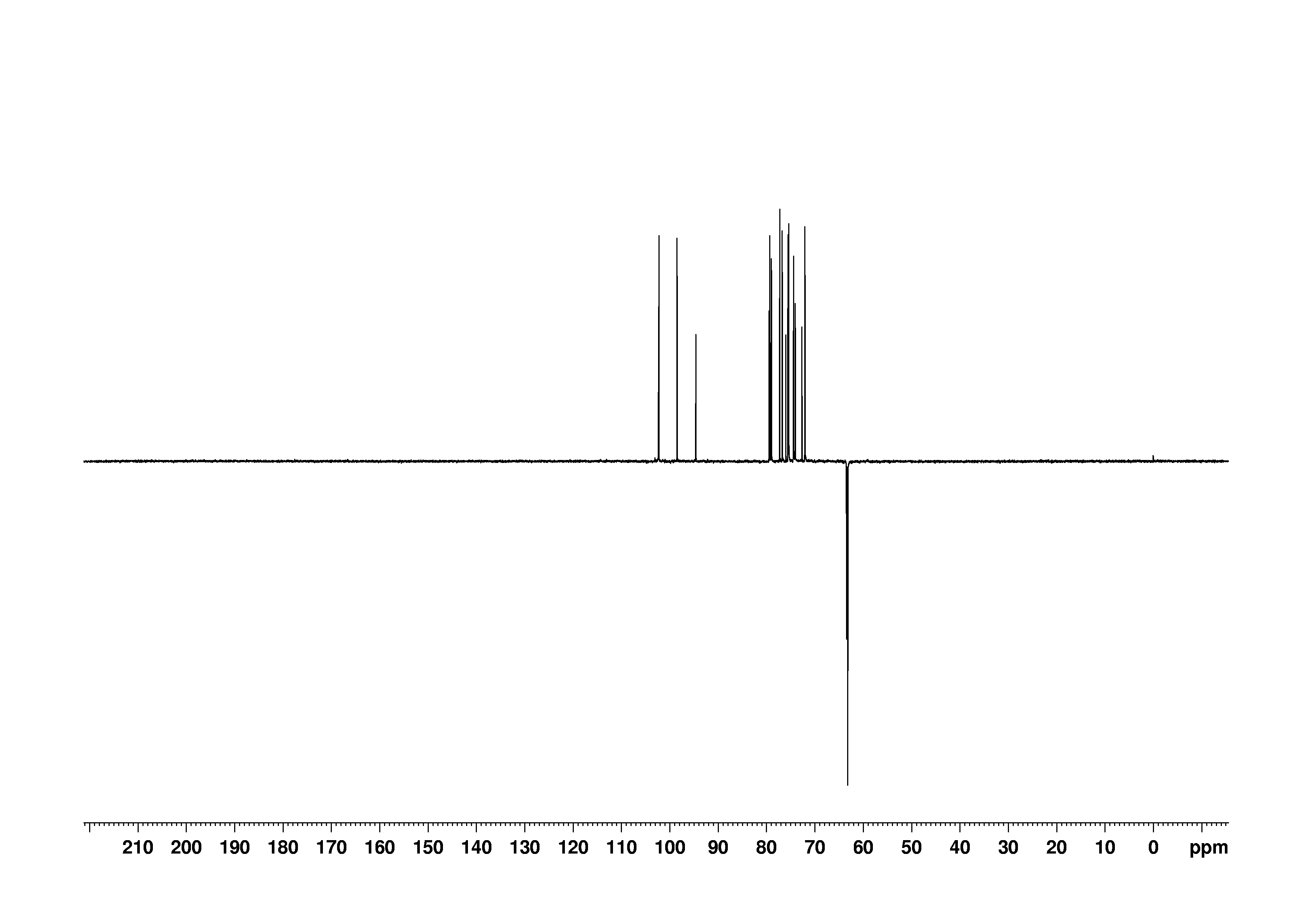
8: 2D [1H,13C]-HSQC
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #8: 2D [1H,13C]-HSQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000017/nmr/set01/spectra/1H_13C_HSQC/00.png)
9: 2D [1H,13C]-HMBC
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #9: 2D [1H,13C]-HMBC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000017/nmr/set01/spectra/1H_13C_HMBC/00.png)
10: 2D [1H,1H]-COSY
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #10: 2D [1H,1H]-COSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000017/nmr/set01/spectra/HH_COSY/00.png)
11: 2D [1H,13C]-HSQC SW small
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
