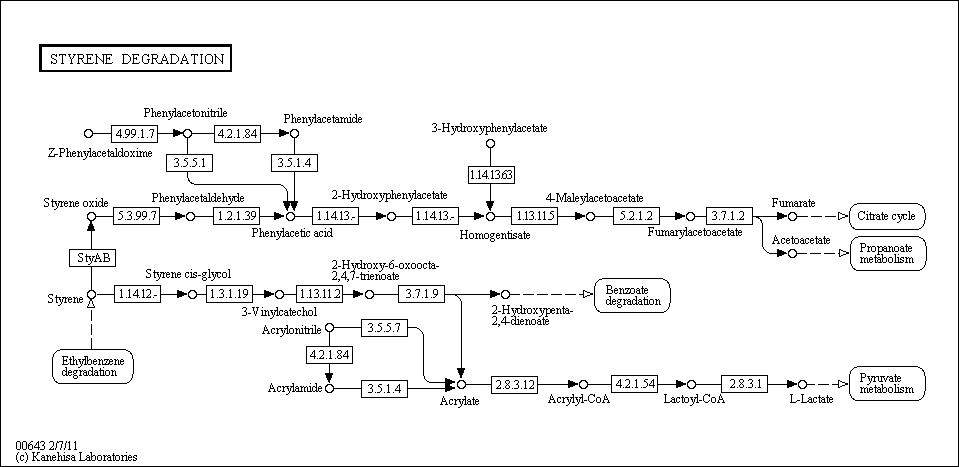
Styrene Degredation
Description: When styrene is heated, exposed to light, or in the presence of a peroxide catalyst, it undergoes polymerization to form polystyrene, a versatile material used in the manufacture of plastics, synthetic rubber, thermal insulation, and packaging. Although polystyrene has many beneficial commercial applications, the styrene monomer is a classified mutagen and a suspected carcinogen. It is highly toxic to aquatic life, although its half-life is less than two days in water. There are several microorganisms that are capable of initiating styrene biodegradation, and metabolism proceeds via one of two known pathways; the principal reaction may either be an ethylene side-chain monooxygenation or a 2,3-dioxygenation. Source: University of Minnesota
Related BMRB Molecules
- Acrylamide
- Fumaric Acid
- Homogentisic Acid
- 2-Hydroxyphenylacetic Acid
- 3-Hydroxyphenylacetic Acid
- L-Lactic Acid
- Phenylacetic Acid
For complete information about pathway, see KEGG [map00643]
