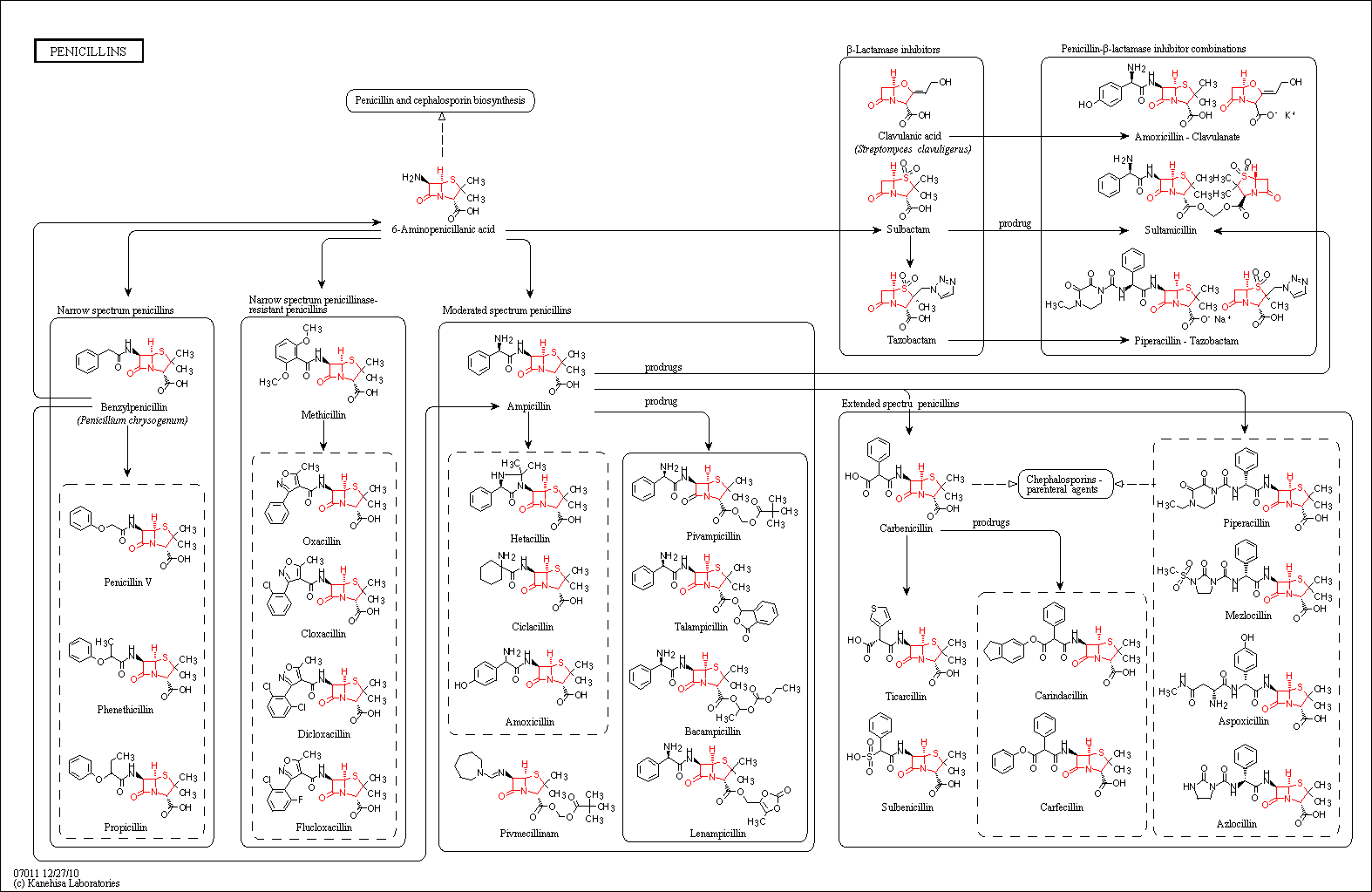
Penicillins
Description: Penicillins are a class of antibiotics originally derived from the Penicillium fungi. They were first discovered by Alexander Fleming in 1928. At the time, this was a very important discovery as they could be used to treat such bacterial infections as syphilis and those caused by staphyloccus and streptococcus which at the time were very serious and often lethal. Today, the general term 'penicillin' can refer to benzylpenicillin (penicillin G), procaine benzylpenicillin, benzathine benzylpenicillin, or phenoxymethylpenicillin (penicillin V). All these drugs are still used today, primarily against Gram-positive bacteria.
These antibiotics work by disrupting the synthesis of this peptidoglycan layer of the bacterial cell wall. In the final step of the formation, normally transpeptidases bind to the ends of muropeptides (intermediates in the formation of peptidoglycan) in order to crosslink the peptidogylcan. Cephalosporins - as well as other beta-lactam antibiotics - act to competitively inhibit this crosslinking by mimicking the binding site on the muropeptide. The build up of these peptidoglycan precursors can trigger activation of cell wall hydrolases which further break down the cell's protective layer. This can lead to cell death due to osmotic pressure, since water can easily flow through the cell membrane through simple diffusion.
Related BMRB Molecules
For complete information about pathway, see KEGG [map07011]
