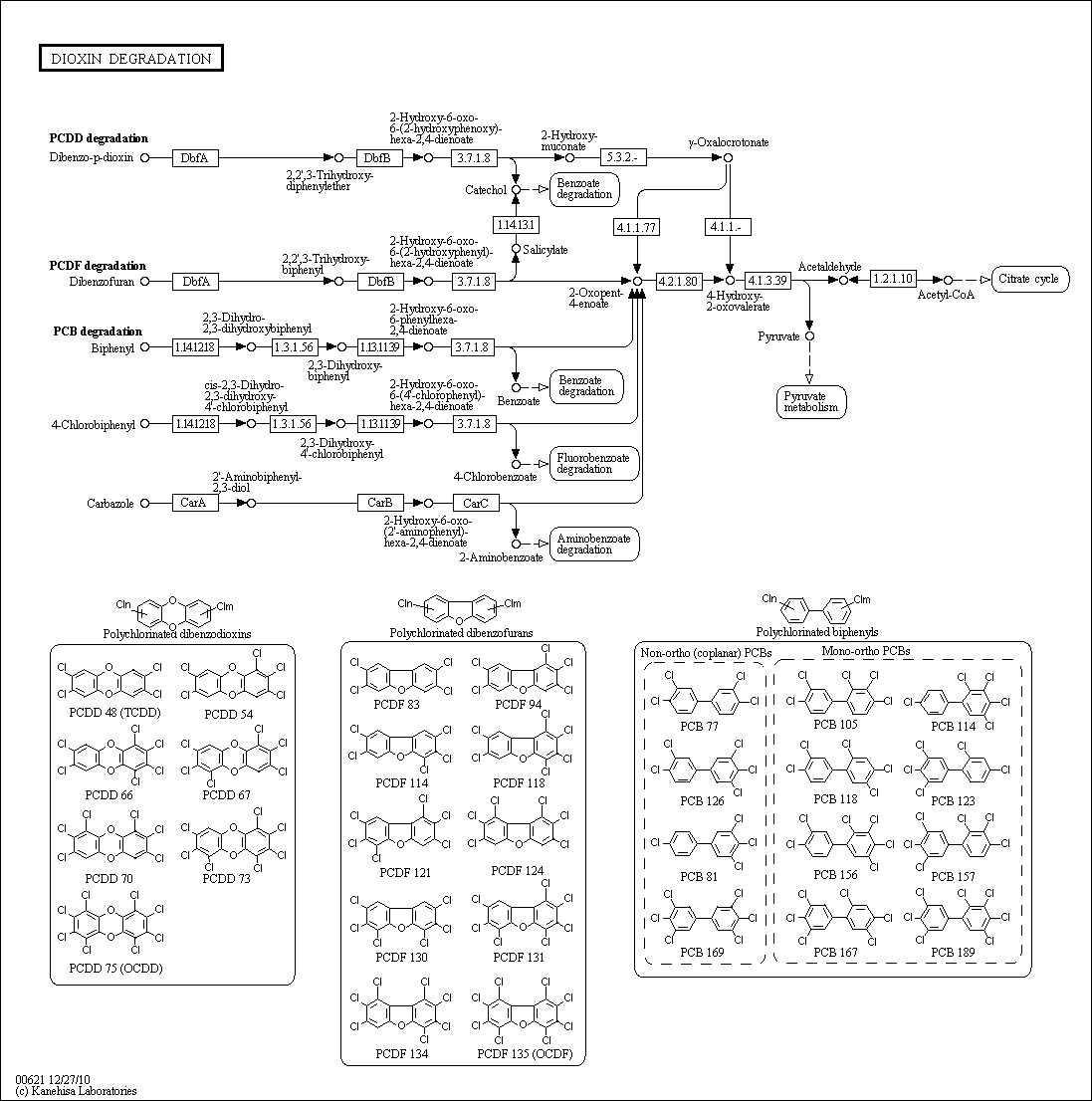
Dioxin Degradation
Description: Dioxins are also referred to as polychlorinated dibenzodioxins (PCDDs) because most all dioxins have multiple chlorine substituents. The most common polychlorinated dioxin is 2,3,7,8-Tetrachlorodibenzodioxin (2,3,7,8-TCDD). Dioxins with chlorines in those four positions have been considered to be toxic. The problem with dioxins is their ability to bioaccumulate within animals and humans. Along with that and their lipophilic properties, it can be considered a carcinogen in humans leading to cell mutation.
One way to degrade dioxin is to incinerate the compounds at a certain temperature. For most organic compounds, complete destruction occurs above 1000 degrees celcius for around two seconds. A temperature between 800-1000 degrees celcius is typically sufficient for a pure sample of 2,3,7,8-TCDD. If the sample of 2,3,7,8-TCDD is impure, the temperature must stay above 800 degrees during the incineration because more TCDDs will form at temperatures around 300-500 degrees celcius.
There are many ways in which dioxins can be degraded into less toxic material but not neccessarily degraded completely. Most of them involve the dehalogenation of the chlorine substituents that are located on the molecule. Some of these processes included are photolysis, radiolysis and ozonolysis.
Related BMRB Molecules
- Anthranilic Acid
- Benzoate
- Biphenyl
- Carbazole
- 4-Chlorobenzoic Acid
- Dibenzofuran
- 4-Methyl-2-Oxovaleric Acid
- Pyrocatechol
- Pyruvic Acid
- Salicylate
For complete information about pathway, see KEGG [map00621]
