Miconazole (C18H14N2OCl4)
BMRB entry bmse000924
Entry DOI: doi:10.13018/BMSE000924
Data source: National Magnetic Facility at Madison - Francisca Jofre, Mark E. Anderson, John L. Markley
NMR-STAR file:
bmse000924.strNMR-STAR
interactive viewerStructure file (mol/sdf):
bmse000924.molAll files for
bmse000924Time Domain Data:
bmse000924.zipSample and instrument details are given with the spectrum
Assigned chemical shifts
Set 1
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
| Atom ID | Author Nomenclature | Value | Ambiguity Code |
|---|---|---|---|
| C18 | C8 | 75.473 | 1 |
| C9 | C9 | 51.658 | 1 |
| C15 | C10 | 134.010 | 4 |
| C15 | C10 | 133.603 | 4 |
| C15 | C10 | 133.380 | 4 |
| C15 | C10 | 133.351 | 4 |
| C15 | C10 | 133.315 | 4 |
| C15 | C10 | 133.113 | 4 |
| C15 | C10 | 129.225 | 4 |
| C15 | C10 | 129.191 | 4 |
| C15 | C10 | 128.619 | 4 |
| C15 | C10 | 128.019 | 4 |
| C15 | C10 | 127.318 | 4 |
| C15 | C10 | 119.680 | 4 |
| C10 | C11 | 67.330 | 1 |
| C17 | C12 | 134.010 | 4 |
| C17 | C12 | 133.603 | 4 |
| C17 | C12 | 133.380 | 4 |
| C17 | C12 | 133.351 | 4 |
| C17 | C12 | 133.315 | 4 |
| C17 | C12 | 133.113 | 4 |
| C17 | C12 | 129.225 | 4 |
| C17 | C12 | 129.191 | 4 |
| C17 | C12 | 128.619 | 4 |
| C17 | C12 | 128.019 | 4 |
| C17 | C12 | 127.318 | 4 |
| C17 | C12 | 119.680 | 4 |
| C4 | C13 | 134.010 | 4 |
| C4 | C13 | 133.603 | 4 |
| C4 | C13 | 133.380 | 4 |
| C4 | C13 | 133.351 | 4 |
| C4 | C13 | 133.315 | 4 |
| C4 | C13 | 133.113 | 4 |
| C4 | C13 | 129.225 | 4 |
| C4 | C13 | 129.191 | 4 |
| C4 | C13 | 128.619 | 4 |
| C4 | C13 | 128.019 | 4 |
| C4 | C13 | 127.318 | 4 |
| C4 | C13 | 119.680 | 4 |
| C12 | C14 | 134.010 | 4 |
| C12 | C14 | 133.603 | 4 |
| C12 | C14 | 133.380 | 4 |
| C12 | C14 | 133.351 | 4 |
| C12 | C14 | 133.315 | 4 |
| C12 | C14 | 133.113 | 4 |
| C12 | C14 | 129.225 | 4 |
| C12 | C14 | 129.191 | 4 |
| C12 | C14 | 128.619 | 4 |
| C12 | C14 | 128.019 | 4 |
| C12 | C14 | 127.318 | 4 |
| C12 | C14 | 119.680 | 4 |
| C6 | C15 | 122.717 | 1 |
| C11 | C16 | 136.120 | 1 |
| C8 | C17 | 134.010 | 4 |
| C8 | C17 | 133.603 | 4 |
| C8 | C17 | 133.380 | 4 |
| C8 | C17 | 133.351 | 4 |
| C8 | C17 | 133.315 | 4 |
| C8 | C17 | 133.113 | 4 |
| C8 | C17 | 129.225 | 4 |
| C8 | C17 | 129.191 | 4 |
| C8 | C17 | 128.619 | 4 |
| C8 | C17 | 128.019 | 4 |
| C8 | C17 | 127.318 | 4 |
| C8 | C17 | 119.680 | 4 |
| C3 | C18 | 134.010 | 4 |
| C3 | C18 | 133.603 | 4 |
| C3 | C18 | 133.380 | 4 |
| C3 | C18 | 133.351 | 4 |
| C3 | C18 | 133.315 | 4 |
| C3 | C18 | 133.113 | 4 |
| C3 | C18 | 129.225 | 4 |
| C3 | C18 | 129.191 | 4 |
| C3 | C18 | 128.619 | 4 |
| C3 | C18 | 128.019 | 4 |
| C3 | C18 | 127.318 | 4 |
| C3 | C18 | 119.680 | 4 |
| C14 | C19 | 134.010 | 4 |
| C14 | C19 | 133.603 | 4 |
| C14 | C19 | 133.380 | 4 |
| C14 | C19 | 133.351 | 4 |
| C14 | C19 | 133.315 | 4 |
| C14 | C19 | 133.113 | 4 |
| C14 | C19 | 129.225 | 4 |
| C14 | C19 | 129.191 | 4 |
| C14 | C19 | 128.619 | 4 |
| C14 | C19 | 128.019 | 4 |
| C14 | C19 | 127.318 | 4 |
| C14 | C19 | 119.680 | 4 |
| C5 | C20 | 134.010 | 4 |
| C5 | C20 | 133.603 | 4 |
| C5 | C20 | 133.380 | 4 |
| C5 | C20 | 133.351 | 4 |
| C5 | C20 | 133.315 | 4 |
| C5 | C20 | 133.113 | 4 |
| C5 | C20 | 129.225 | 4 |
| C5 | C20 | 129.191 | 4 |
| C5 | C20 | 128.619 | 4 |
| C5 | C20 | 128.019 | 4 |
| C5 | C20 | 127.318 | 4 |
| C5 | C20 | 119.680 | 4 |
| C16 | C21 | 134.010 | 4 |
| C16 | C21 | 133.603 | 4 |
| C16 | C21 | 133.380 | 4 |
| C16 | C21 | 133.351 | 4 |
| C16 | C21 | 133.315 | 4 |
| C16 | C21 | 133.113 | 4 |
| C16 | C21 | 129.225 | 4 |
| C16 | C21 | 129.191 | 4 |
| C16 | C21 | 128.619 | 4 |
| C16 | C21 | 128.019 | 4 |
| C16 | C21 | 127.318 | 4 |
| C16 | C21 | 119.680 | 4 |
| C1 | C22 | 131.192 | 1 |
| C7 | C23 | 134.010 | 4 |
| C7 | C23 | 133.603 | 4 |
| C7 | C23 | 133.380 | 4 |
| C7 | C23 | 133.351 | 4 |
| C7 | C23 | 133.315 | 4 |
| C7 | C23 | 133.113 | 4 |
| C7 | C23 | 129.225 | 4 |
| C7 | C23 | 129.191 | 4 |
| C7 | C23 | 128.619 | 4 |
| C7 | C23 | 128.019 | 4 |
| C7 | C23 | 127.318 | 4 |
| C7 | C23 | 119.680 | 4 |
| C2 | C24 | 134.010 | 4 |
| C2 | C24 | 133.603 | 4 |
| C2 | C24 | 133.380 | 4 |
| C2 | C24 | 133.351 | 4 |
| C2 | C24 | 133.315 | 4 |
| C2 | C24 | 133.113 | 4 |
| C2 | C24 | 129.225 | 4 |
| C2 | C24 | 129.191 | 4 |
| C2 | C24 | 128.619 | 4 |
| C2 | C24 | 128.019 | 4 |
| C2 | C24 | 127.318 | 4 |
| C2 | C24 | 119.680 | 4 |
| C13 | C25 | 134.010 | 4 |
| C13 | C25 | 133.603 | 4 |
| C13 | C25 | 133.380 | 4 |
| C13 | C25 | 133.351 | 4 |
| C13 | C25 | 133.315 | 4 |
| C13 | C25 | 133.113 | 4 |
| C13 | C25 | 129.225 | 4 |
| C13 | C25 | 129.191 | 4 |
| C13 | C25 | 128.619 | 4 |
| C13 | C25 | 128.019 | 4 |
| C13 | C25 | 127.318 | 4 |
| C13 | C25 | 119.680 | 4 |
| H39 | H26 | 5.209 | 1 |
| H34 | H27 | 4.551 | 1 |
| H35 | H28 | 4.551 | 1 |
| H36 | H29 | 4.456 | 1 |
| H37 | H30 | 4.456 | 1 |
| H29 | H31 | 7.432 | 4 |
| H29 | H31 | 7.751 | 4 |
| H29 | H31 | 7.687 | 4 |
| H29 | H31 | 7.604 | 4 |
| H29 | H31 | 7.546 | 4 |
| H31 | H32 | 7.663 | 1 |
| H38 | H33 | 9.075 | 1 |
| H33 | H34 | 7.432 | 4 |
| H33 | H34 | 7.751 | 4 |
| H33 | H34 | 7.687 | 4 |
| H33 | H34 | 7.604 | 4 |
| H33 | H34 | 7.546 | 4 |
| H28 | H35 | 7.432 | 4 |
| H28 | H35 | 7.751 | 4 |
| H28 | H35 | 7.687 | 4 |
| H28 | H35 | 7.604 | 4 |
| H28 | H35 | 7.546 | 4 |
| H30 | H36 | 7.432 | 4 |
| H30 | H36 | 7.751 | 4 |
| H30 | H36 | 7.687 | 4 |
| H30 | H36 | 7.604 | 4 |
| H30 | H36 | 7.546 | 4 |
| H26 | H37 | 7.432 | 4 |
| H26 | H37 | 7.751 | 4 |
| H26 | H37 | 7.687 | 4 |
| H26 | H37 | 7.604 | 4 |
| H26 | H37 | 7.546 | 4 |
| H32 | H38 | 7.432 | 4 |
| H32 | H38 | 7.751 | 4 |
| H32 | H38 | 7.687 | 4 |
| H32 | H38 | 7.604 | 4 |
| H32 | H38 | 7.546 | 4 |
| H27 | H39 | 7.432 | 4 |
| H27 | H39 | 7.751 | 4 |
| H27 | H39 | 7.687 | 4 |
| H27 | H39 | 7.604 | 4 |
| H27 | H39 | 7.546 | 4 |
NMR experiments
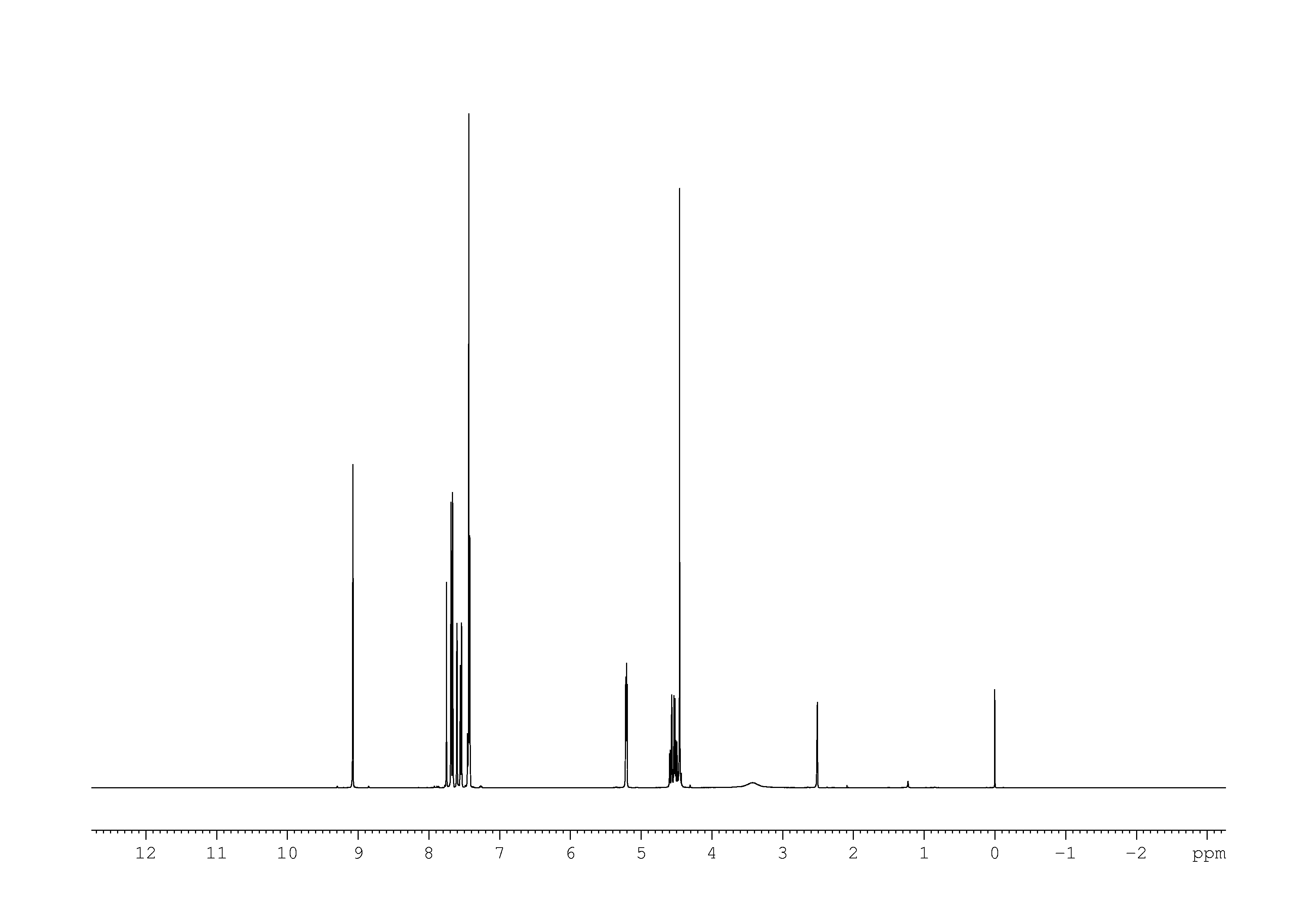
1: 1D 1H
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
2: 2D [1H,1H]-TOCSY
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #2: 2D [1H,1H]-TOCSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000924/nmr/set01/spectra/HH_TOCSY/00.png)
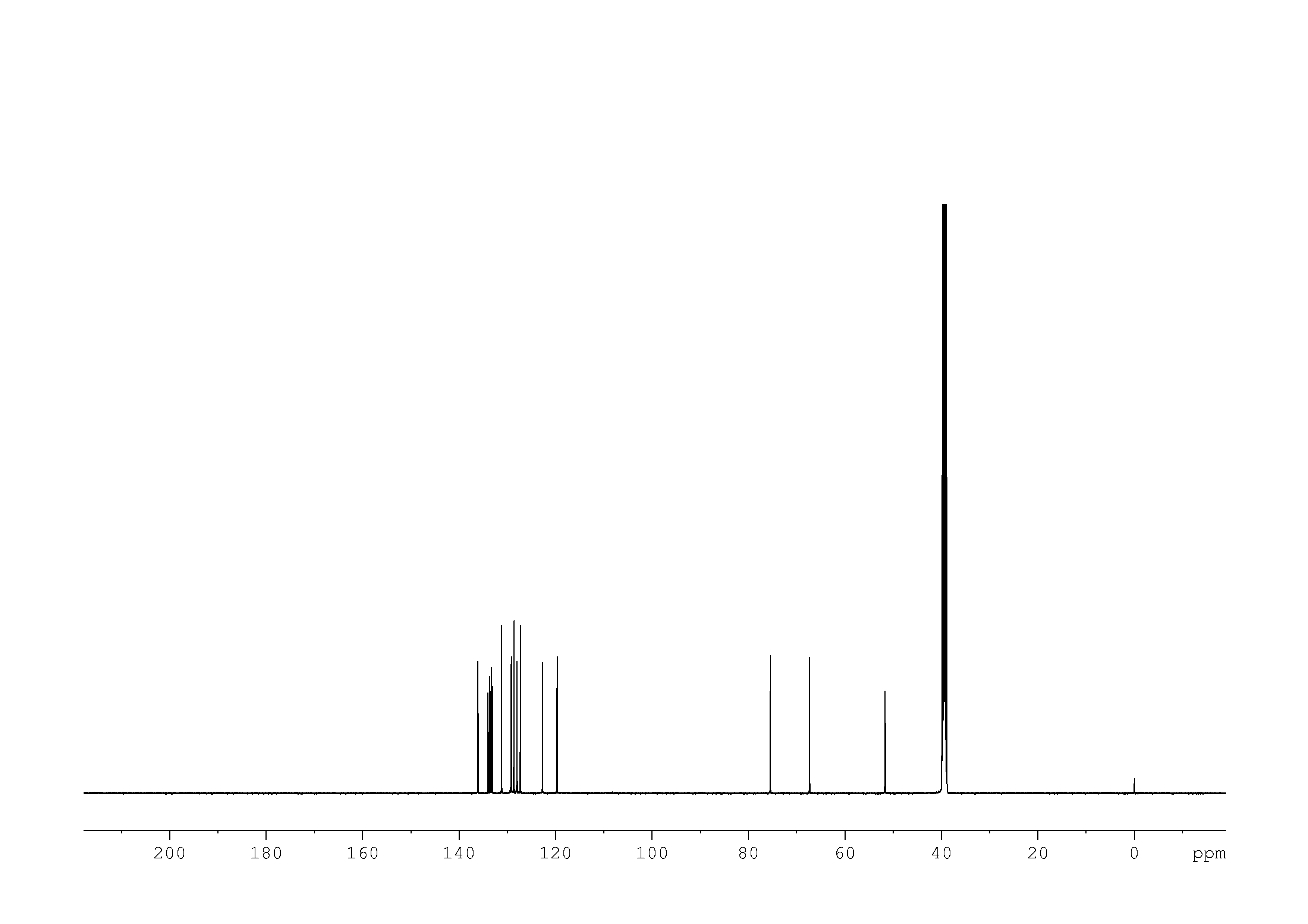
3: 1D 13C
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
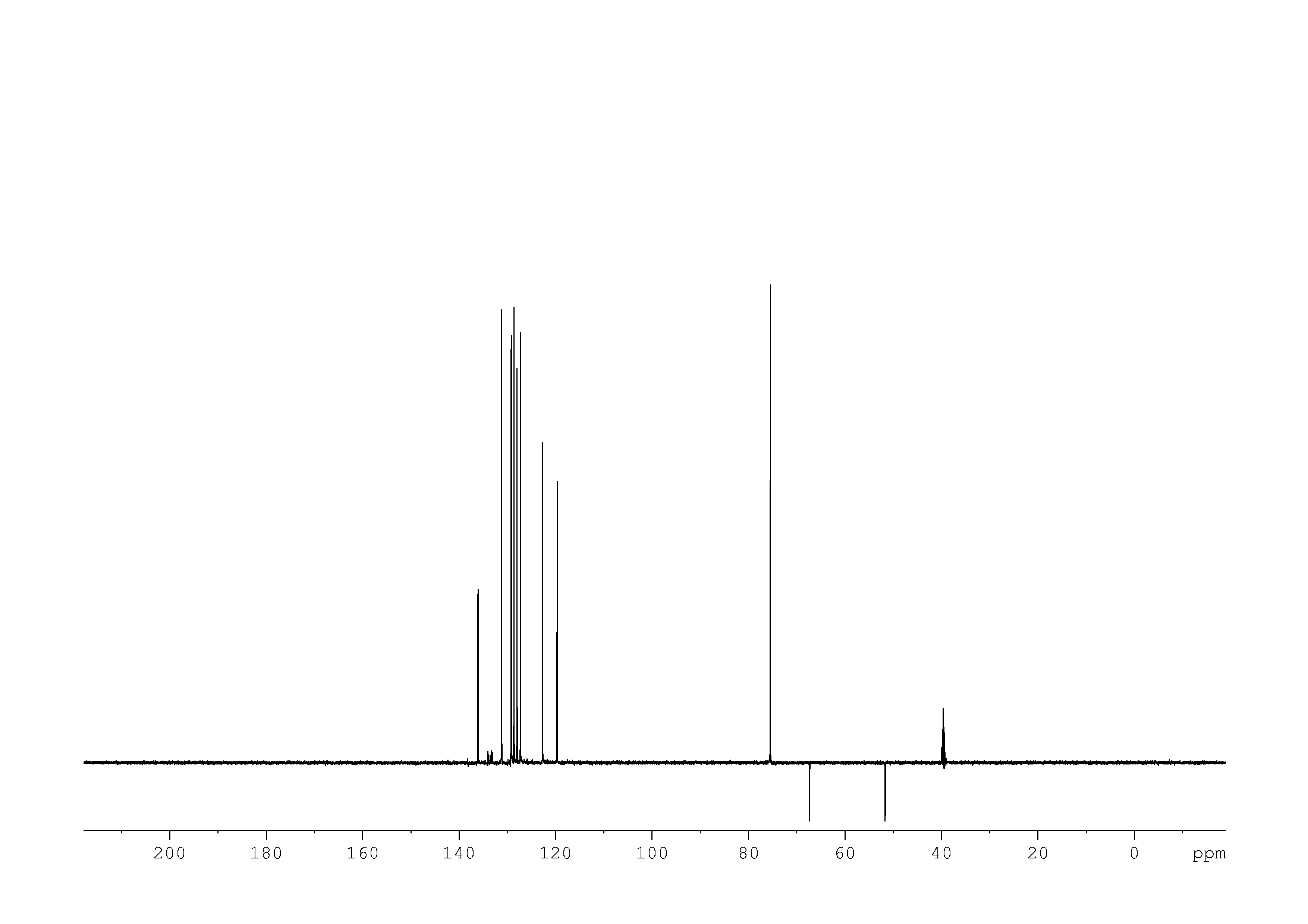
4: 1D DEPT90
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
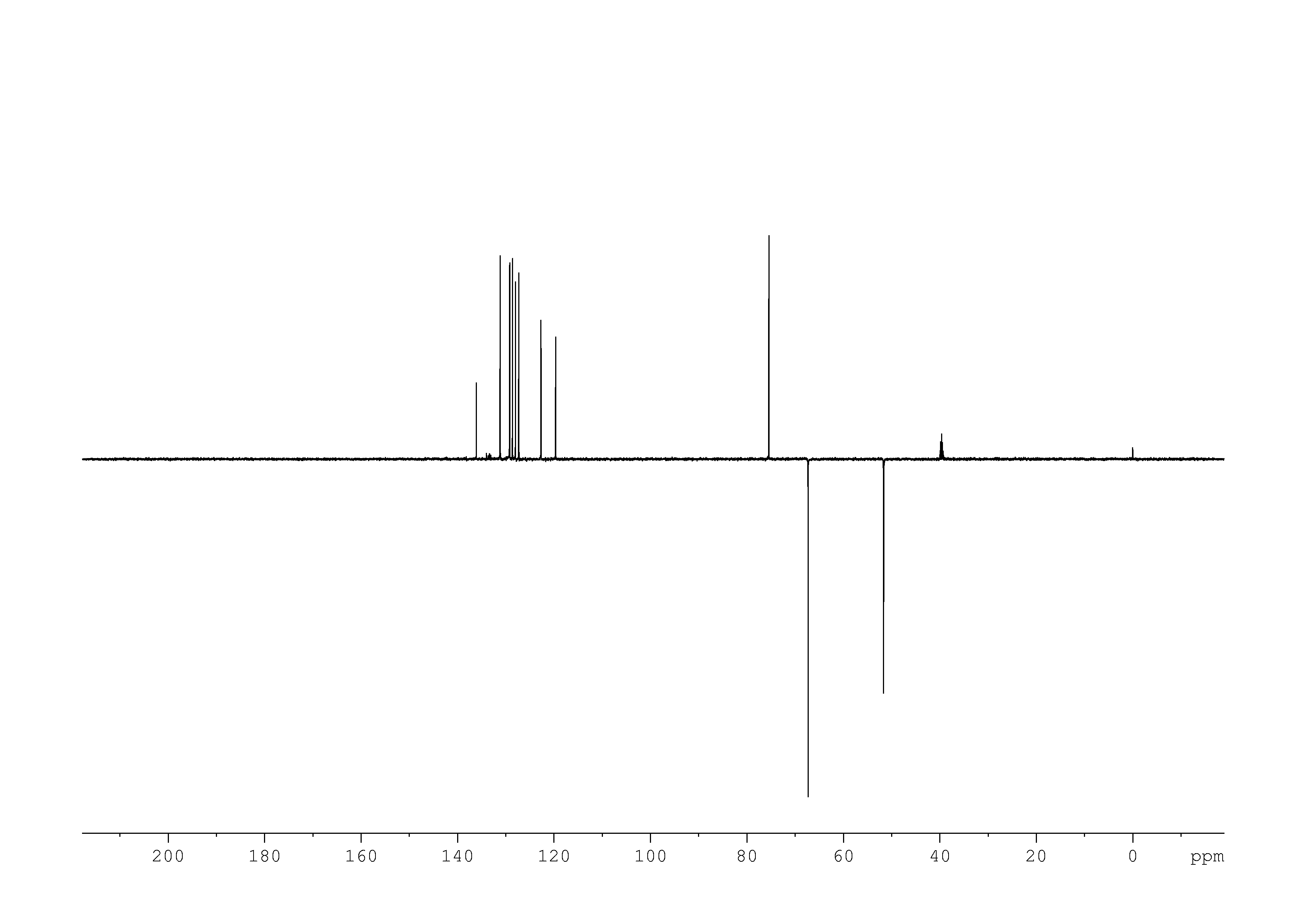
5: 1D DEPT135
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
6: 2D [1H,13C]-HSQC
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #6: 2D [1H,13C]-HSQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000924/nmr/set01/spectra/1H_13C_HSQC/00.png)
7: 2D [1H,13C]-HMBC
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #7: 2D [1H,13C]-HMBC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000924/nmr/set01/spectra/1H_13C_HMBC/00.png)
8: 2D [1H,1H]-COSY
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #8: 2D [1H,1H]-COSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000924/nmr/set01/spectra/HH_COSY/00.png)
9: 2D [1H,13C]-HMQC
Sample: 51mM in DMSO, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #9: 2D [1H,13C]-HMQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000924/nmr/set01/spectra/1H_13C_HMQC/00.png)
