2-alpha-Mannobiose (C12H22O11)
BMRB entry bmse000910
Entry DOI: doi:10.13018/BMSE000910
Data source: National Magnetic Facility at Madison - Francisca Jofre, Mark E. Anderson, John L. Markley, Melanie E. Ulrich
NMR-STAR file:
bmse000910.strNMR-STAR
interactive viewerStructure file (mol/sdf):
bmse000910.molAll files for
bmse000910Time Domain Data:
bmse000910.zipSample and instrument details are given with the spectrum
Assigned chemical shifts
Set 1
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
| Atom ID | Author Nomenclature | Value | Ambiguity Code |
|---|---|---|---|
| C10 | C12 | 96.559 | 4 |
| C10 | C12 | 96.422 | 4 |
| C10 | C12 | 79.570 | 4 |
| C10 | C12 | 79.257 | 4 |
| C10 | C12 | 79.158 | 4 |
| C10 | C12 | 77.520 | 4 |
| C10 | C12 | 75.508 | 4 |
| C10 | C12 | 74.434 | 4 |
| C10 | C12 | 73.600 | 4 |
| C10 | C12 | 73.347 | 4 |
| C10 | C12 | 73.258 | 4 |
| C10 | C12 | 72.911 | 4 |
| C10 | C12 | 71.684 | 4 |
| C10 | C12 | 69.428 | 4 |
| C8 | C13 | 96.559 | 4 |
| C8 | C13 | 96.422 | 4 |
| C8 | C13 | 79.570 | 4 |
| C8 | C13 | 79.257 | 4 |
| C8 | C13 | 79.158 | 4 |
| C8 | C13 | 77.520 | 4 |
| C8 | C13 | 75.508 | 4 |
| C8 | C13 | 74.434 | 4 |
| C8 | C13 | 73.600 | 4 |
| C8 | C13 | 73.347 | 4 |
| C8 | C13 | 73.258 | 4 |
| C8 | C13 | 72.911 | 4 |
| C8 | C13 | 71.684 | 4 |
| C8 | C13 | 69.428 | 4 |
| C12 | C14 | 102.941 | 1 |
| C12 | C14 | 102.899 | 1 |
| C9 | C15 | 96.559 | 4 |
| C9 | C15 | 96.422 | 4 |
| C9 | C15 | 79.570 | 4 |
| C9 | C15 | 79.257 | 4 |
| C9 | C15 | 79.158 | 4 |
| C9 | C15 | 77.520 | 4 |
| C9 | C15 | 75.508 | 4 |
| C9 | C15 | 74.434 | 4 |
| C9 | C15 | 73.600 | 4 |
| C9 | C15 | 73.347 | 4 |
| C9 | C15 | 73.258 | 4 |
| C9 | C15 | 72.911 | 4 |
| C9 | C15 | 71.684 | 4 |
| C9 | C15 | 69.428 | 4 |
| C7 | C16 | 96.559 | 4 |
| C7 | C16 | 96.422 | 4 |
| C7 | C16 | 79.570 | 4 |
| C7 | C16 | 79.257 | 4 |
| C7 | C16 | 79.158 | 4 |
| C7 | C16 | 77.520 | 4 |
| C7 | C16 | 75.508 | 4 |
| C7 | C16 | 74.434 | 4 |
| C7 | C16 | 73.600 | 4 |
| C7 | C16 | 73.347 | 4 |
| C7 | C16 | 73.258 | 4 |
| C7 | C16 | 72.911 | 4 |
| C7 | C16 | 71.684 | 4 |
| C7 | C16 | 69.428 | 4 |
| C6 | C17 | 96.559 | 4 |
| C6 | C17 | 96.422 | 4 |
| C6 | C17 | 79.570 | 4 |
| C6 | C17 | 79.257 | 4 |
| C6 | C17 | 79.158 | 4 |
| C6 | C17 | 77.520 | 4 |
| C6 | C17 | 75.508 | 4 |
| C6 | C17 | 74.434 | 4 |
| C6 | C17 | 73.600 | 4 |
| C6 | C17 | 73.347 | 4 |
| C6 | C17 | 73.258 | 4 |
| C6 | C17 | 72.911 | 4 |
| C6 | C17 | 71.684 | 4 |
| C6 | C17 | 69.428 | 4 |
| C5 | C18 | 96.559 | 4 |
| C5 | C18 | 96.422 | 4 |
| C5 | C18 | 79.570 | 4 |
| C5 | C18 | 79.257 | 4 |
| C5 | C18 | 79.158 | 4 |
| C5 | C18 | 77.520 | 4 |
| C5 | C18 | 75.508 | 4 |
| C5 | C18 | 74.434 | 4 |
| C5 | C18 | 73.600 | 4 |
| C5 | C18 | 73.347 | 4 |
| C5 | C18 | 73.258 | 4 |
| C5 | C18 | 72.911 | 4 |
| C5 | C18 | 71.684 | 4 |
| C5 | C18 | 69.428 | 4 |
| C4 | C19 | 96.559 | 4 |
| C4 | C19 | 96.422 | 4 |
| C4 | C19 | 79.570 | 4 |
| C4 | C19 | 79.257 | 4 |
| C4 | C19 | 79.158 | 4 |
| C4 | C19 | 77.520 | 4 |
| C4 | C19 | 75.508 | 4 |
| C4 | C19 | 74.434 | 4 |
| C4 | C19 | 73.600 | 4 |
| C4 | C19 | 73.347 | 4 |
| C4 | C19 | 73.258 | 4 |
| C4 | C19 | 72.911 | 4 |
| C4 | C19 | 71.684 | 4 |
| C4 | C19 | 69.428 | 4 |
| C3 | C20 | 96.559 | 4 |
| C3 | C20 | 96.422 | 4 |
| C3 | C20 | 79.570 | 4 |
| C3 | C20 | 79.257 | 4 |
| C3 | C20 | 79.158 | 4 |
| C3 | C20 | 77.520 | 4 |
| C3 | C20 | 75.508 | 4 |
| C3 | C20 | 74.434 | 4 |
| C3 | C20 | 73.600 | 4 |
| C3 | C20 | 73.347 | 4 |
| C3 | C20 | 73.258 | 4 |
| C3 | C20 | 72.911 | 4 |
| C3 | C20 | 71.684 | 4 |
| C3 | C20 | 69.428 | 4 |
| C11 | C21 | 96.559 | 4 |
| C11 | C21 | 96.422 | 4 |
| C11 | C21 | 79.570 | 4 |
| C11 | C21 | 79.257 | 4 |
| C11 | C21 | 79.158 | 4 |
| C11 | C21 | 77.520 | 4 |
| C11 | C21 | 75.508 | 4 |
| C11 | C21 | 74.434 | 4 |
| C11 | C21 | 73.600 | 4 |
| C11 | C21 | 73.347 | 4 |
| C11 | C21 | 73.258 | 4 |
| C11 | C21 | 72.911 | 4 |
| C11 | C21 | 71.684 | 4 |
| C11 | C21 | 69.428 | 4 |
| C2 | C22 | 63.749 | 4 |
| C2 | C22 | 63.218 | 4 |
| C2 | C22 | 57.094 | 4 |
| C1 | C23 | 63.749 | 4 |
| C1 | C23 | 63.218 | 4 |
| C1 | C23 | 57.094 | 4 |
| H35 | H24 | 5.174 | 4 |
| H35 | H24 | 4.907 | 4 |
| H35 | H24 | 4.062 | 4 |
| H35 | H24 | 3.850 | 4 |
| H35 | H24 | 3.654 | 4 |
| H35 | H24 | 3.559 | 4 |
| H35 | H24 | 3.496 | 4 |
| H35 | H24 | 3.434 | 4 |
| H33 | H25 | 5.174 | 4 |
| H33 | H25 | 4.907 | 4 |
| H33 | H25 | 4.062 | 4 |
| H33 | H25 | 3.850 | 4 |
| H33 | H25 | 3.654 | 4 |
| H33 | H25 | 3.559 | 4 |
| H33 | H25 | 3.496 | 4 |
| H33 | H25 | 3.434 | 4 |
| H37 | H26 | 4.734 | 1 |
| H34 | H27 | 5.174 | 4 |
| H34 | H27 | 4.907 | 4 |
| H34 | H27 | 4.062 | 4 |
| H34 | H27 | 3.850 | 4 |
| H34 | H27 | 3.654 | 4 |
| H34 | H27 | 3.559 | 4 |
| H34 | H27 | 3.496 | 4 |
| H34 | H27 | 3.434 | 4 |
| H32 | H28 | 5.174 | 4 |
| H32 | H28 | 4.907 | 4 |
| H32 | H28 | 4.062 | 4 |
| H32 | H28 | 3.850 | 4 |
| H32 | H28 | 3.654 | 4 |
| H32 | H28 | 3.559 | 4 |
| H32 | H28 | 3.496 | 4 |
| H32 | H28 | 3.434 | 4 |
| H31 | H29 | 5.174 | 4 |
| H31 | H29 | 4.907 | 4 |
| H31 | H29 | 4.062 | 4 |
| H31 | H29 | 3.850 | 4 |
| H31 | H29 | 3.654 | 4 |
| H31 | H29 | 3.559 | 4 |
| H31 | H29 | 3.496 | 4 |
| H31 | H29 | 3.434 | 4 |
| H30 | H30 | 5.174 | 4 |
| H30 | H30 | 4.907 | 4 |
| H30 | H30 | 4.062 | 4 |
| H30 | H30 | 3.850 | 4 |
| H30 | H30 | 3.654 | 4 |
| H30 | H30 | 3.559 | 4 |
| H30 | H30 | 3.496 | 4 |
| H30 | H30 | 3.434 | 4 |
| H29 | H31 | 5.174 | 4 |
| H29 | H31 | 4.907 | 4 |
| H29 | H31 | 4.062 | 4 |
| H29 | H31 | 3.850 | 4 |
| H29 | H31 | 3.654 | 4 |
| H29 | H31 | 3.559 | 4 |
| H29 | H31 | 3.496 | 4 |
| H29 | H31 | 3.434 | 4 |
| H28 | H32 | 5.174 | 4 |
| H28 | H32 | 4.907 | 4 |
| H28 | H32 | 4.062 | 4 |
| H28 | H32 | 3.850 | 4 |
| H28 | H32 | 3.654 | 4 |
| H28 | H32 | 3.559 | 4 |
| H28 | H32 | 3.496 | 4 |
| H28 | H32 | 3.434 | 4 |
NMR experiments
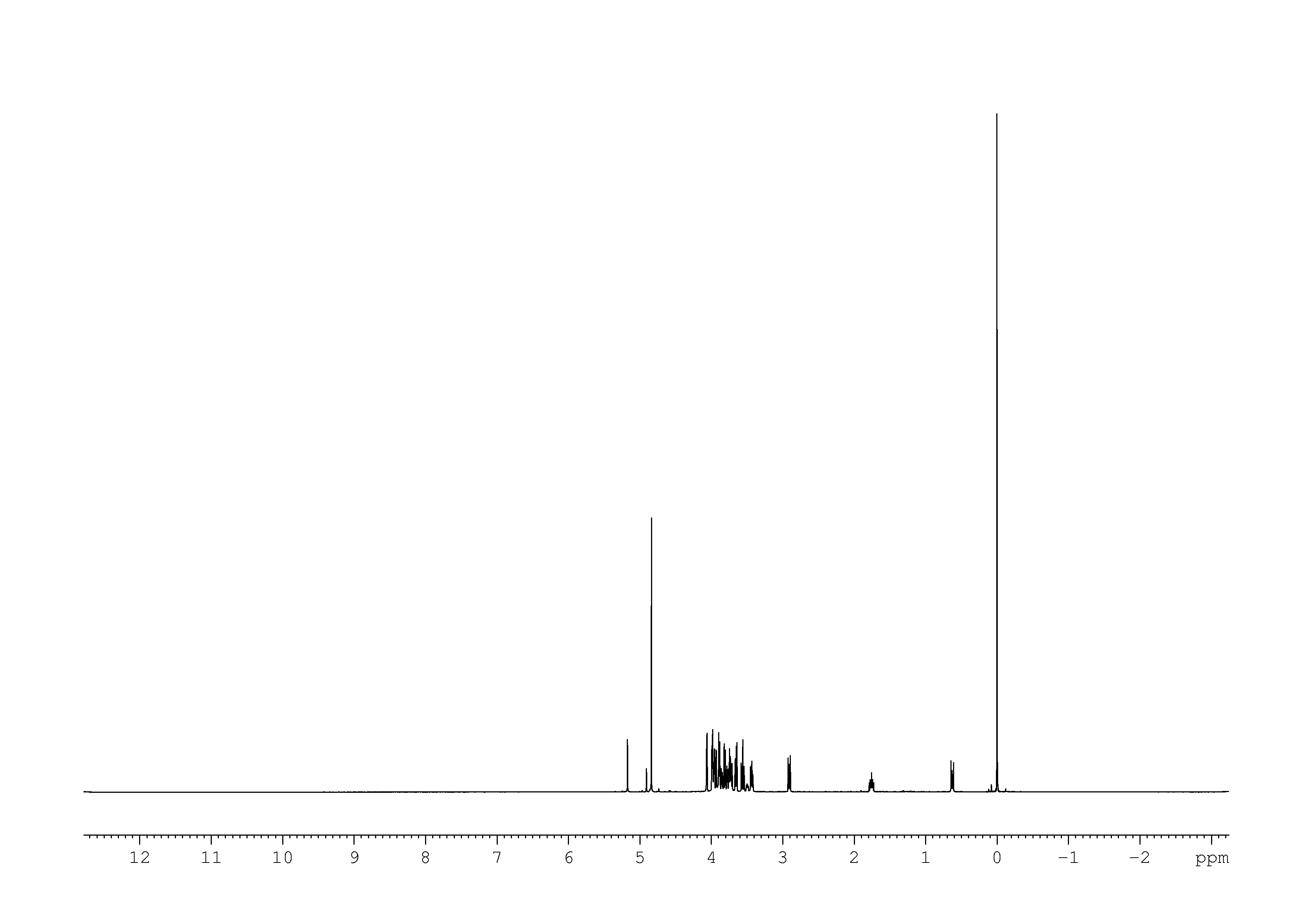
1: 1D 1H
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
2: 2D [1H,1H]-TOCSY
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #2: 2D [1H,1H]-TOCSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000910/nmr/set01/spectra/HH_TOCSY/00.png)
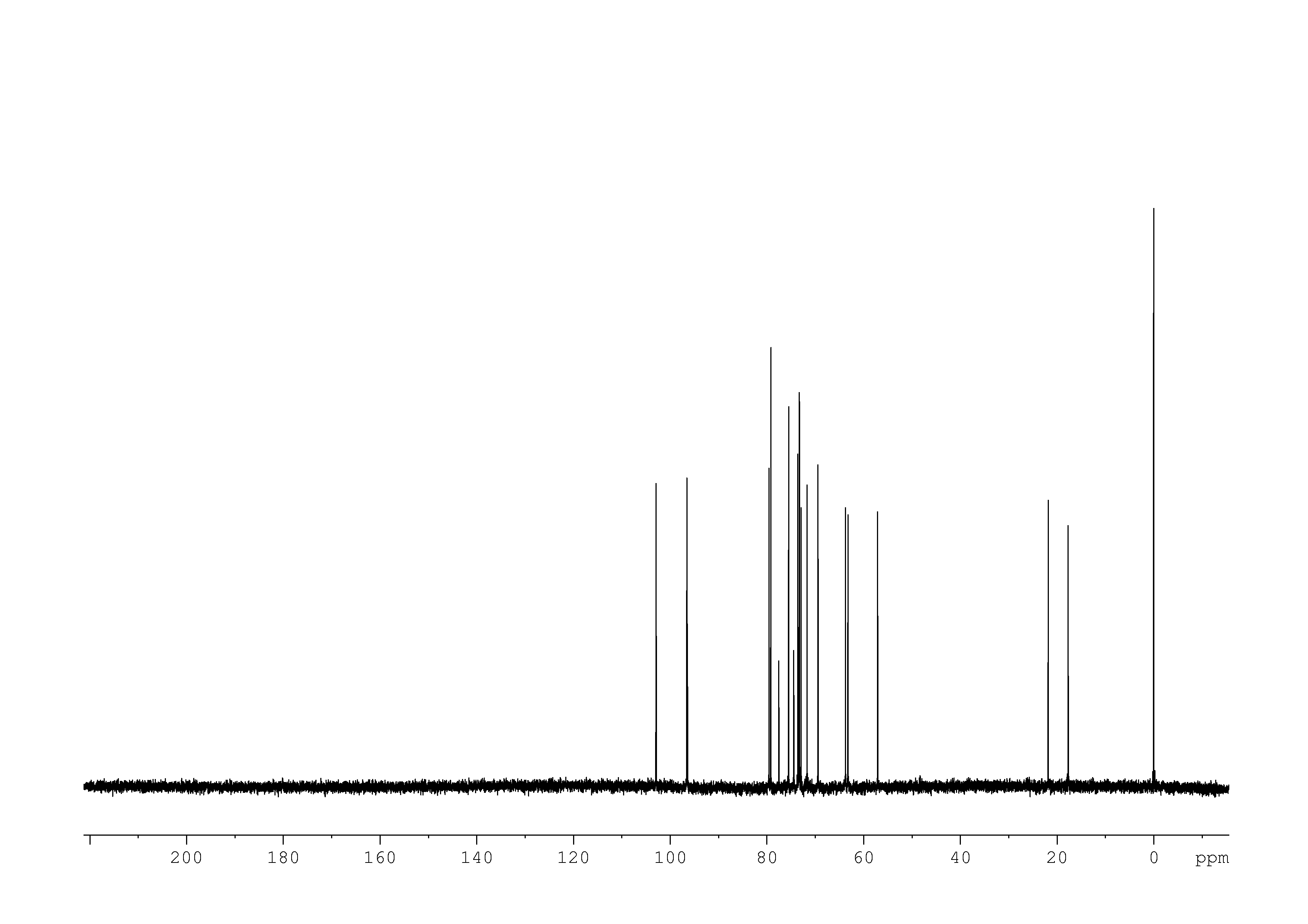
3: 1D 13C
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
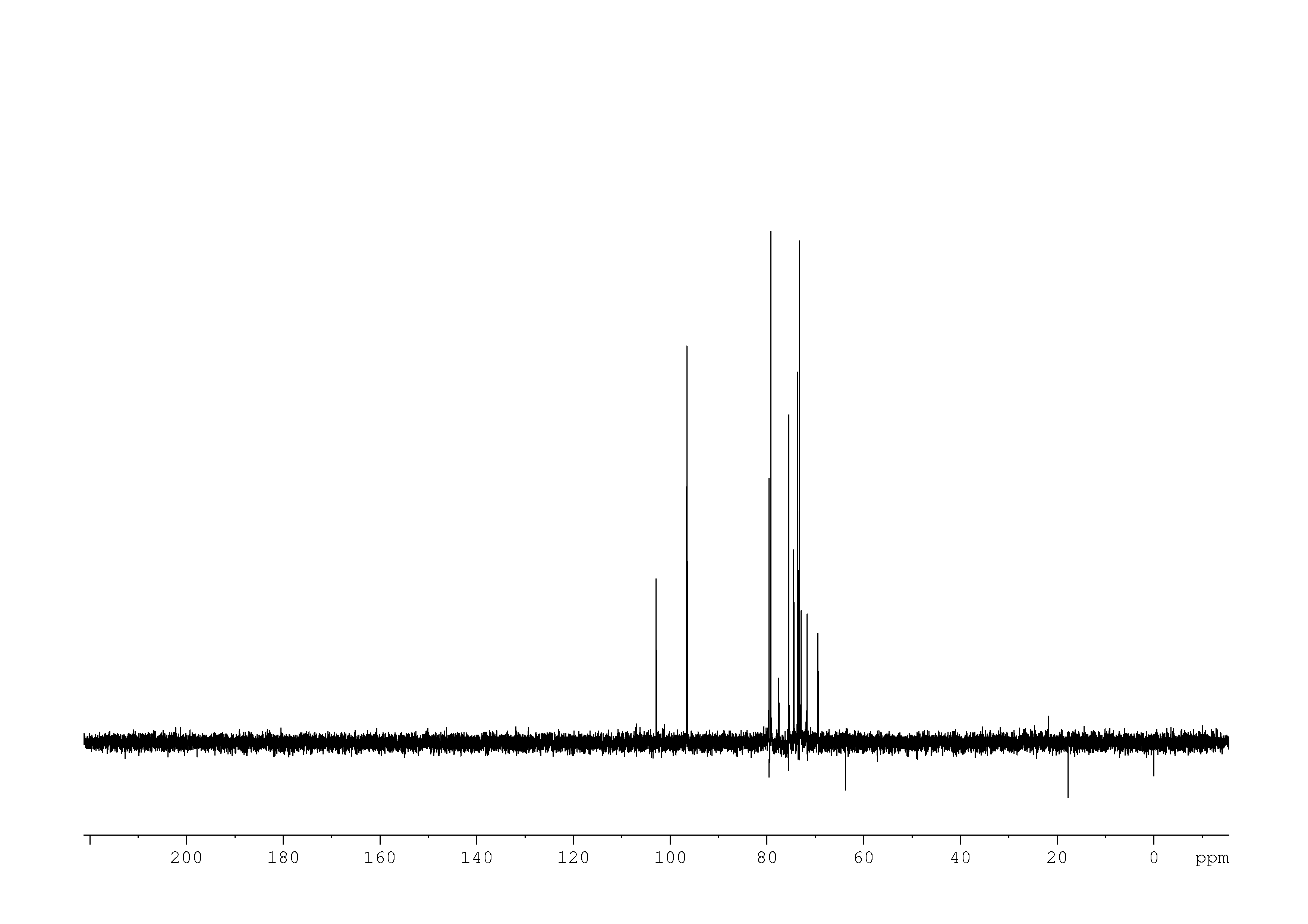
4: 1D DEPT90
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
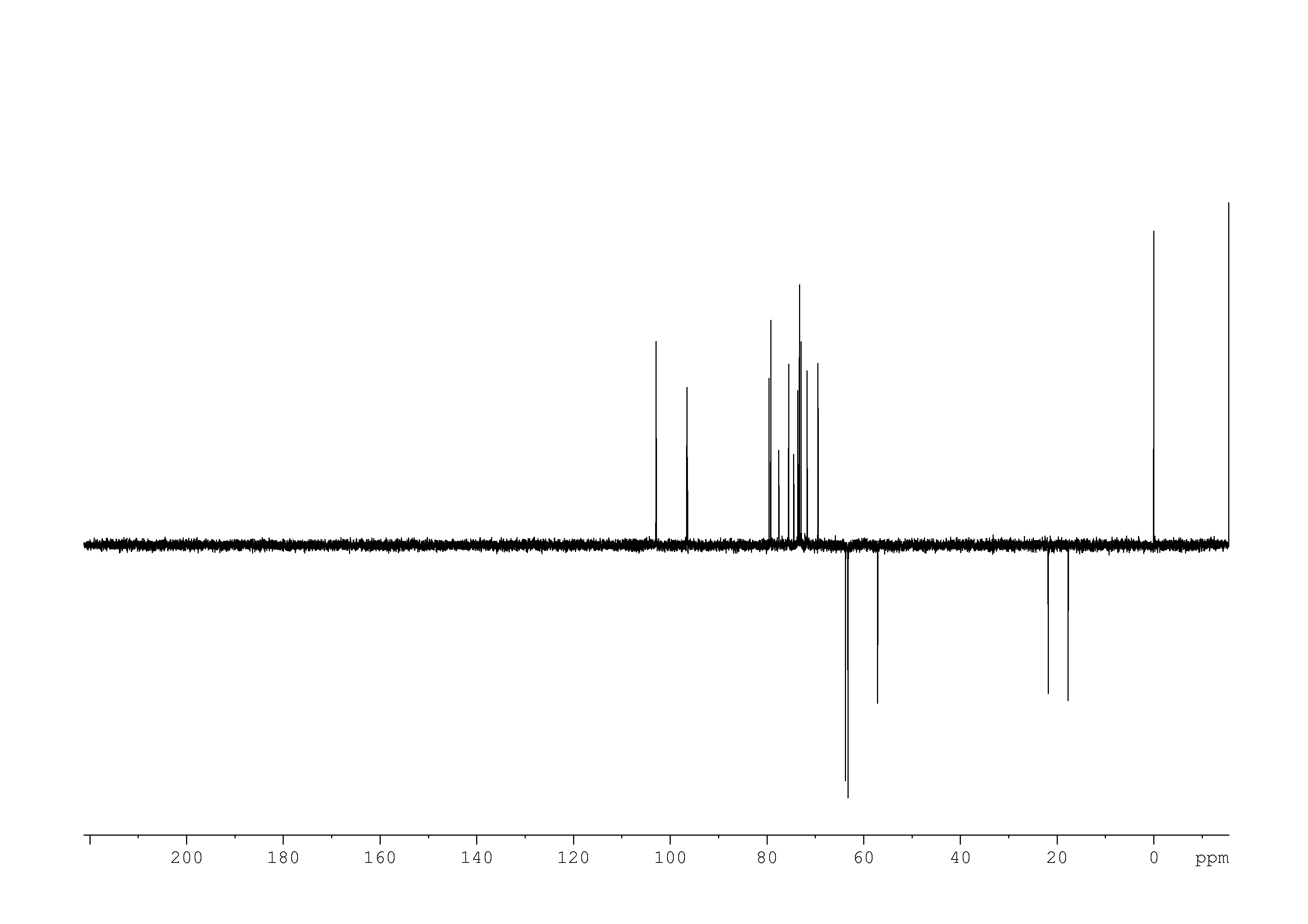
5: 1D DEPT135
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
6: 2D [1H,13C]-HSQC
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #6: 2D [1H,13C]-HSQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000910/nmr/set01/spectra/1H_13C_HSQC/00.png)
7: 2D [1H,13C]-HMBC
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #7: 2D [1H,13C]-HMBC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000910/nmr/set01/spectra/1H_13C_HMBC/00.png)
8: 2D [1H,1H]-COSY
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #8: 2D [1H,1H]-COSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000910/nmr/set01/spectra/HH_COSY/00.png)
9: 2D [1H,13C]-HMQC
Sample: 10mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #9: 2D [1H,13C]-HMQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000910/nmr/set01/spectra/1H_13C_HMQC/00.png)
