Digoxigenin (C23H34O5)
BMRB entry bmse000735
Entry DOI: doi:10.13018/BMSE000735
Data source: Madison Metabolomics Consortium - Francisca Jofre, Mark E. Anderson, John L. Markley, Ravi Rapolu
NMR-STAR file:
bmse000735.strNMR-STAR
interactive viewerStructure file (mol/sdf):
bmse000735.molAll files for
bmse000735Time Domain Data:
bmse000735.zipSample and instrument details are given with the spectrum
Assigned chemical shifts
Set 1
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
| Atom ID | Author Nomenclature | Value | Ambiguity Code |
|---|---|---|---|
| C7 | C7 | 86.861 | 1 |
| C8 | C8 | 42.165 | 1 |
| C9 | C9 | 57.309 | 1 |
| C10 | C10 | 37.469 | 4 |
| C10 | C10 | 34.113 | 4 |
| C10 | C10 | 33.509 | 4 |
| C10 | C10 | 33.413 | 4 |
| C10 | C10 | 30.839 | 4 |
| C10 | C10 | 30.814 | 4 |
| C10 | C10 | 28.465 | 4 |
| C10 | C10 | 28.381 | 4 |
| C10 | C10 | 27.797 | 4 |
| C10 | C10 | 22.783 | 4 |
| C11 | C11 | 36.364 | 1 |
| C12 | C12 | 75.677 | 1 |
| C13 | C13 | 37.469 | 4 |
| C13 | C13 | 34.113 | 4 |
| C13 | C13 | 33.509 | 4 |
| C13 | C13 | 33.413 | 4 |
| C13 | C13 | 30.839 | 4 |
| C13 | C13 | 30.814 | 4 |
| C13 | C13 | 28.465 | 4 |
| C13 | C13 | 28.381 | 4 |
| C13 | C13 | 27.797 | 4 |
| C13 | C13 | 22.783 | 4 |
| C14 | C14 | 37.469 | 4 |
| C14 | C14 | 34.113 | 4 |
| C14 | C14 | 33.509 | 4 |
| C14 | C14 | 33.413 | 4 |
| C14 | C14 | 30.839 | 4 |
| C14 | C14 | 30.814 | 4 |
| C14 | C14 | 28.465 | 4 |
| C14 | C14 | 28.381 | 4 |
| C14 | C14 | 27.797 | 4 |
| C14 | C14 | 22.783 | 4 |
| C15 | C15 | 47.041 | 1 |
| C16 | C16 | 37.469 | 4 |
| C16 | C16 | 34.113 | 4 |
| C16 | C16 | 33.509 | 4 |
| C16 | C16 | 33.413 | 4 |
| C16 | C16 | 30.839 | 4 |
| C16 | C16 | 30.814 | 4 |
| C16 | C16 | 28.465 | 4 |
| C16 | C16 | 28.381 | 4 |
| C16 | C16 | 27.797 | 4 |
| C16 | C16 | 22.783 | 4 |
| C17 | C17 | 37.469 | 4 |
| C17 | C17 | 34.113 | 4 |
| C17 | C17 | 33.509 | 4 |
| C17 | C17 | 33.413 | 4 |
| C17 | C17 | 30.839 | 4 |
| C17 | C17 | 30.814 | 4 |
| C17 | C17 | 28.465 | 4 |
| C17 | C17 | 28.381 | 4 |
| C17 | C17 | 27.797 | 4 |
| C17 | C17 | 22.783 | 4 |
| C18 | C18 | 37.469 | 4 |
| C18 | C18 | 34.113 | 4 |
| C18 | C18 | 33.509 | 4 |
| C18 | C18 | 33.413 | 4 |
| C18 | C18 | 30.839 | 4 |
| C18 | C18 | 30.814 | 4 |
| C18 | C18 | 28.465 | 4 |
| C18 | C18 | 28.381 | 4 |
| C18 | C18 | 27.797 | 4 |
| C18 | C18 | 22.783 | 4 |
| C19 | C19 | 37.469 | 4 |
| C19 | C19 | 34.113 | 4 |
| C19 | C19 | 33.509 | 4 |
| C19 | C19 | 33.413 | 4 |
| C19 | C19 | 30.839 | 4 |
| C19 | C19 | 30.814 | 4 |
| C19 | C19 | 28.465 | 4 |
| C19 | C19 | 28.381 | 4 |
| C19 | C19 | 27.797 | 4 |
| C19 | C19 | 22.783 | 4 |
| C20 | C20 | 37.469 | 4 |
| C20 | C20 | 34.113 | 4 |
| C20 | C20 | 33.509 | 4 |
| C20 | C20 | 33.413 | 4 |
| C20 | C20 | 30.839 | 4 |
| C20 | C20 | 30.814 | 4 |
| C20 | C20 | 28.465 | 4 |
| C20 | C20 | 28.381 | 4 |
| C20 | C20 | 27.797 | 4 |
| C20 | C20 | 22.783 | 4 |
| C21 | C21 | 9.931 | 1 |
| C22 | C22 | 37.469 | 4 |
| C22 | C22 | 34.113 | 4 |
| C22 | C22 | 33.509 | 4 |
| C22 | C22 | 33.413 | 4 |
| C22 | C22 | 30.839 | 4 |
| C22 | C22 | 30.814 | 4 |
| C22 | C22 | 28.465 | 4 |
| C22 | C22 | 28.381 | 4 |
| C22 | C22 | 27.797 | 4 |
| C22 | C22 | 22.783 | 4 |
| C23 | C23 | 24.255 | 1 |
| C24 | C24 | 37.469 | 4 |
| C24 | C24 | 34.113 | 4 |
| C24 | C24 | 33.509 | 4 |
| C24 | C24 | 33.413 | 4 |
| C24 | C24 | 30.839 | 4 |
| C24 | C24 | 30.814 | 4 |
| C24 | C24 | 28.465 | 4 |
| C24 | C24 | 28.381 | 4 |
| C24 | C24 | 27.797 | 4 |
| C24 | C24 | 22.783 | 4 |
| C25 | C25 | 67.608 | 1 |
| C26 | C26 | 178.56 | 1 |
| C27 | C27 | 75.478 | 1 |
| C28 | C28 | 117.751 | 1 |
| C29 | C29 | 177.365 | 1 |
| H30 | H30 | 2.145 | 4 |
| H30 | H30 | 1.940 | 4 |
| H30 | H30 | 1.750 | 4 |
| H30 | H30 | 1.615 | 4 |
| H30 | H30 | 1.501 | 4 |
| H30 | H30 | 1.284 | 4 |
| H31 | H31 | 2.145 | 4 |
| H31 | H31 | 1.940 | 4 |
| H31 | H31 | 1.750 | 4 |
| H31 | H31 | 1.615 | 4 |
| H31 | H31 | 1.501 | 4 |
| H31 | H31 | 1.284 | 4 |
| H32 | H32 | 3.386 | 1 |
| H33 | H33 | 2.145 | 4 |
| H33 | H33 | 1.940 | 4 |
| H33 | H33 | 1.750 | 4 |
| H33 | H33 | 1.615 | 4 |
| H33 | H33 | 1.501 | 4 |
| H33 | H33 | 1.284 | 4 |
| H34 | H34 | 2.145 | 4 |
| H34 | H34 | 1.940 | 4 |
| H34 | H34 | 1.750 | 4 |
| H34 | H34 | 1.615 | 4 |
| H34 | H34 | 1.501 | 4 |
| H34 | H34 | 1.284 | 4 |
| H35 | H35 | 2.145 | 4 |
| H35 | H35 | 1.940 | 4 |
| H35 | H35 | 1.750 | 4 |
| H35 | H35 | 1.615 | 4 |
| H35 | H35 | 1.501 | 4 |
| H35 | H35 | 1.284 | 4 |
| H36 | H36 | 3.319 | 1 |
| H37 | H37 | 2.145 | 4 |
| H37 | H37 | 1.940 | 4 |
| H37 | H37 | 1.750 | 4 |
| H37 | H37 | 1.615 | 4 |
| H37 | H37 | 1.501 | 4 |
| H37 | H37 | 1.284 | 4 |
| H38 | H38 | 2.145 | 4 |
| H38 | H38 | 1.940 | 4 |
| H38 | H38 | 1.750 | 4 |
| H38 | H38 | 1.615 | 4 |
| H38 | H38 | 1.501 | 4 |
| H38 | H38 | 1.284 | 4 |
| H39 | H39 | 2.145 | 4 |
| H39 | H39 | 1.940 | 4 |
| H39 | H39 | 1.750 | 4 |
| H39 | H39 | 1.615 | 4 |
| H39 | H39 | 1.501 | 4 |
| H39 | H39 | 1.284 | 4 |
| H40 | H40 | 2.145 | 4 |
| H40 | H40 | 1.940 | 4 |
| H40 | H40 | 1.750 | 4 |
| H40 | H40 | 1.615 | 4 |
| H40 | H40 | 1.501 | 4 |
| H40 | H40 | 1.284 | 4 |
| H41 | H41 | 2.145 | 4 |
| H41 | H41 | 1.940 | 4 |
| H41 | H41 | 1.750 | 4 |
| H41 | H41 | 1.615 | 4 |
| H41 | H41 | 1.501 | 4 |
| H41 | H41 | 1.284 | 4 |
| H42 | H42 | 2.145 | 4 |
| H42 | H42 | 1.940 | 4 |
| H42 | H42 | 1.750 | 4 |
| H42 | H42 | 1.615 | 4 |
| H42 | H42 | 1.501 | 4 |
| H42 | H42 | 1.284 | 4 |
| H43 | H43 | 2.145 | 4 |
| H43 | H43 | 1.940 | 4 |
| H43 | H43 | 1.750 | 4 |
| H43 | H43 | 1.615 | 4 |
| H43 | H43 | 1.501 | 4 |
| H43 | H43 | 1.284 | 4 |
| H44 | H44 | 2.145 | 4 |
| H44 | H44 | 1.940 | 4 |
| H44 | H44 | 1.750 | 4 |
| H44 | H44 | 1.615 | 4 |
| H44 | H44 | 1.501 | 4 |
| H44 | H44 | 1.284 | 4 |
| H45 | H45 | 2.145 | 4 |
| H45 | H45 | 1.940 | 4 |
| H45 | H45 | 1.750 | 4 |
| H45 | H45 | 1.615 | 4 |
| H45 | H45 | 1.501 | 4 |
| H45 | H45 | 1.284 | 4 |
| H46 | H46 | 2.145 | 4 |
| H46 | H46 | 1.940 | 4 |
| H46 | H46 | 1.750 | 4 |
| H46 | H46 | 1.615 | 4 |
| H46 | H46 | 1.501 | 4 |
| H46 | H46 | 1.284 | 4 |
| H47 | H47 | 0.782 | 1 |
| H48 | H48 | 0.782 | 1 |
| H49 | H49 | 0.782 | 1 |
| H50 | H50 | 2.145 | 4 |
| H50 | H50 | 1.940 | 4 |
| H50 | H50 | 1.750 | 4 |
| H50 | H50 | 1.615 | 4 |
| H50 | H50 | 1.501 | 4 |
| H50 | H50 | 1.284 | 4 |
| H51 | H51 | 2.145 | 4 |
| H51 | H51 | 1.940 | 4 |
| H51 | H51 | 1.750 | 4 |
| H51 | H51 | 1.615 | 4 |
| H51 | H51 | 1.501 | 4 |
| H51 | H51 | 1.284 | 4 |
| H52 | H52 | 0.974 | 1 |
| H53 | H53 | 0.974 | 1 |
| H54 | H54 | 0.974 | 1 |
| H55 | H55 | 2.145 | 4 |
| H55 | H55 | 1.940 | 4 |
| H55 | H55 | 1.750 | 4 |
| H55 | H55 | 1.615 | 4 |
| H55 | H55 | 1.501 | 4 |
| H55 | H55 | 1.284 | 4 |
| H56 | H56 | 2.145 | 4 |
| H56 | H56 | 1.940 | 4 |
| H56 | H56 | 1.750 | 4 |
| H56 | H56 | 1.615 | 4 |
| H56 | H56 | 1.501 | 4 |
| H56 | H56 | 1.284 | 4 |
| H57 | H57 | 4.049 | 1 |
| H60 | H60 | 4.941 | 1 |
| H61 | H61 | 4.941 | 1 |
| H62 | H62 | 5.903 | 1 |
NMR experiments
1: 1D 1H
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
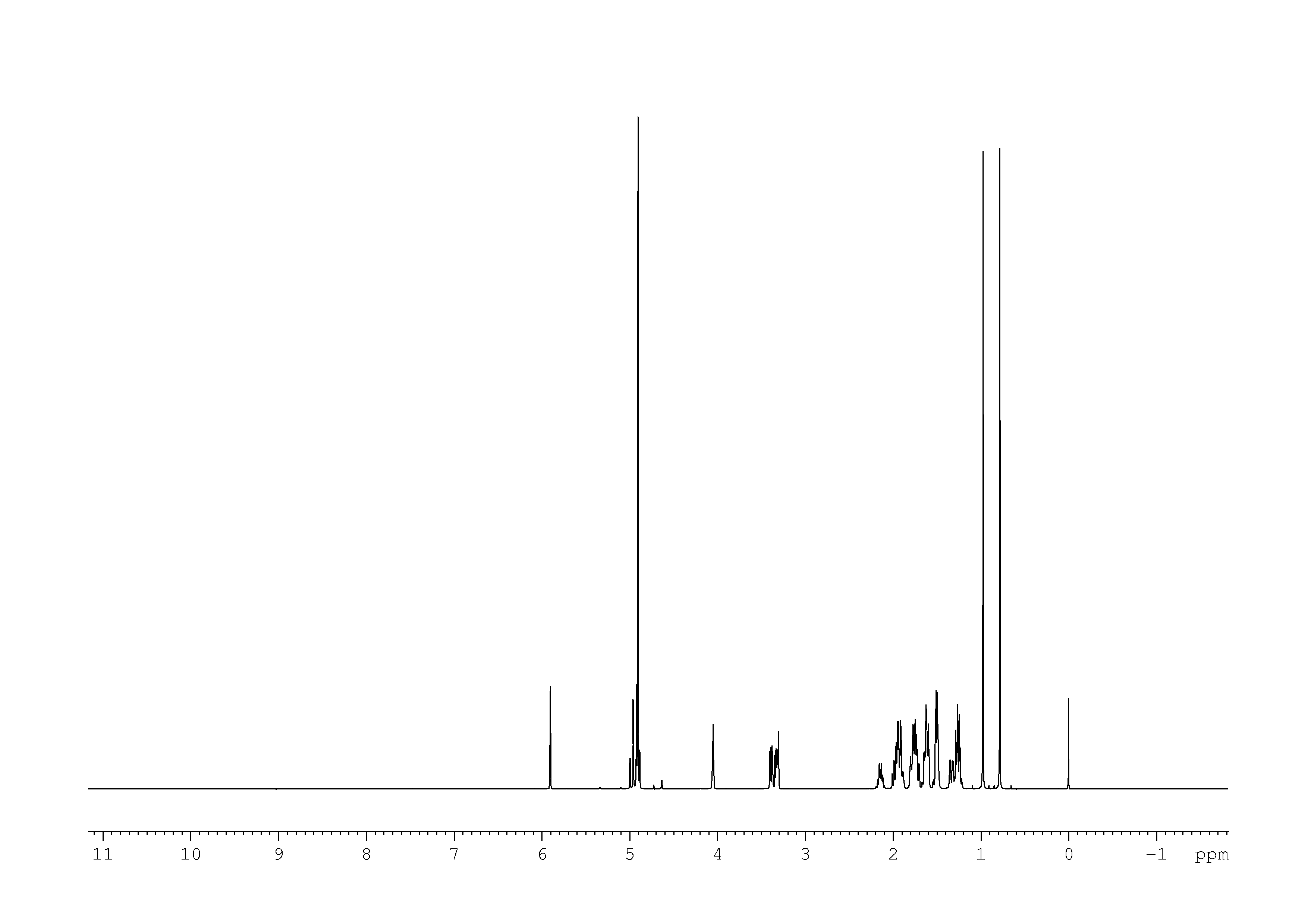
2: 2D [1H,1H]-TOCSY
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #2: 2D [1H,1H]-TOCSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000735/nmr/set01/spectra/HH_TOCSY.png)
3: 1D 13C
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
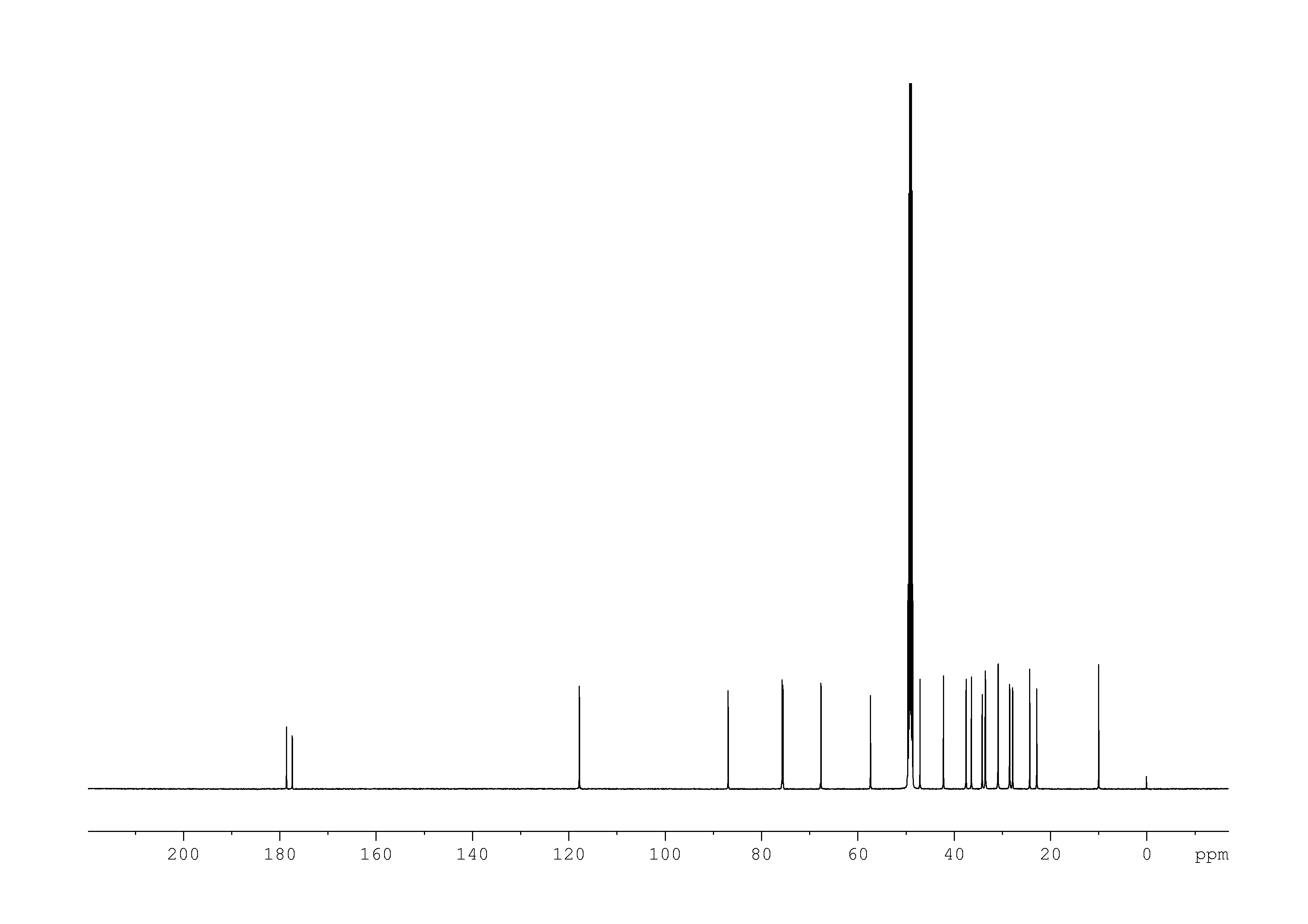
4: 1D DEPT90
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
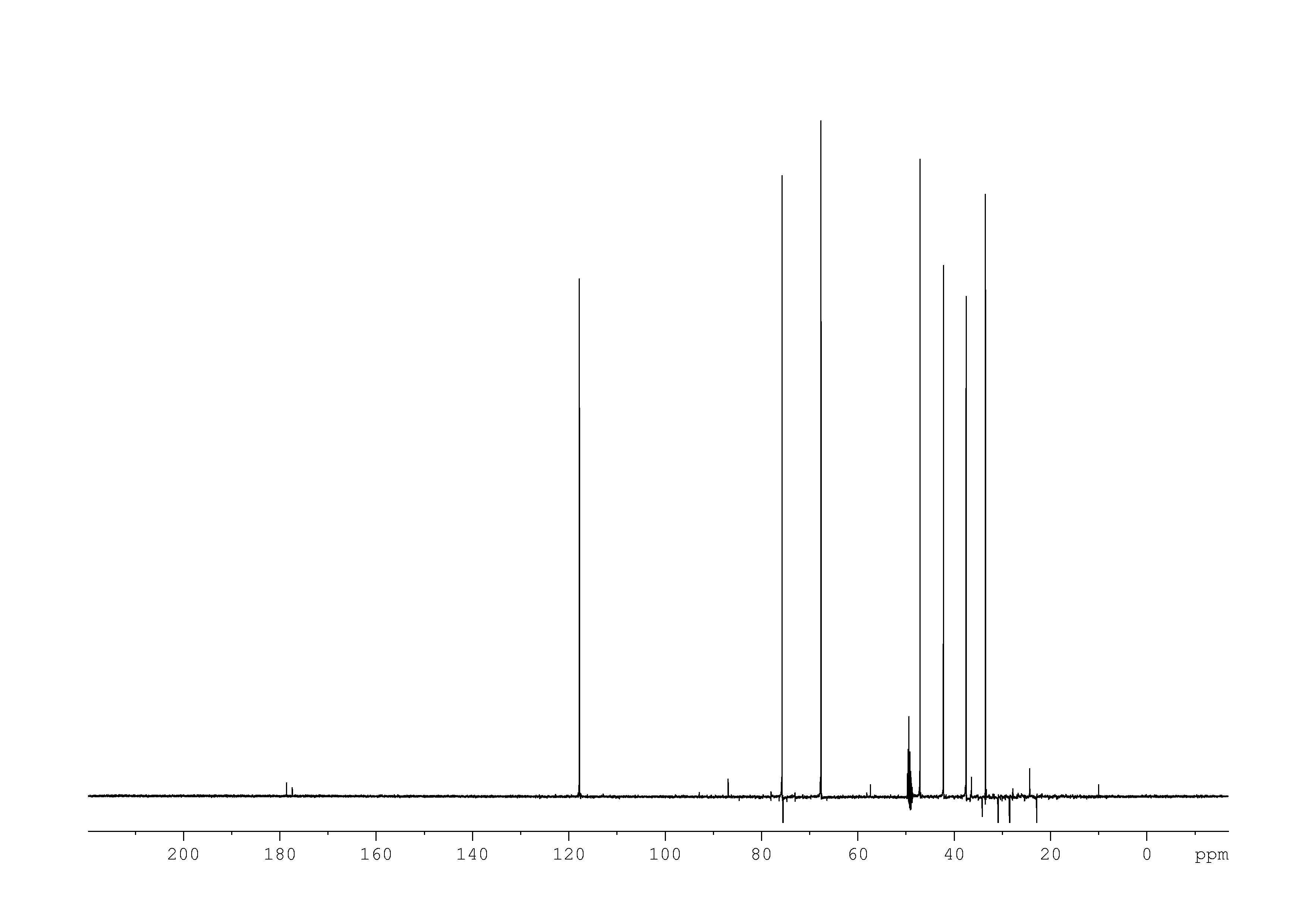
5: 1D DEPT135
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
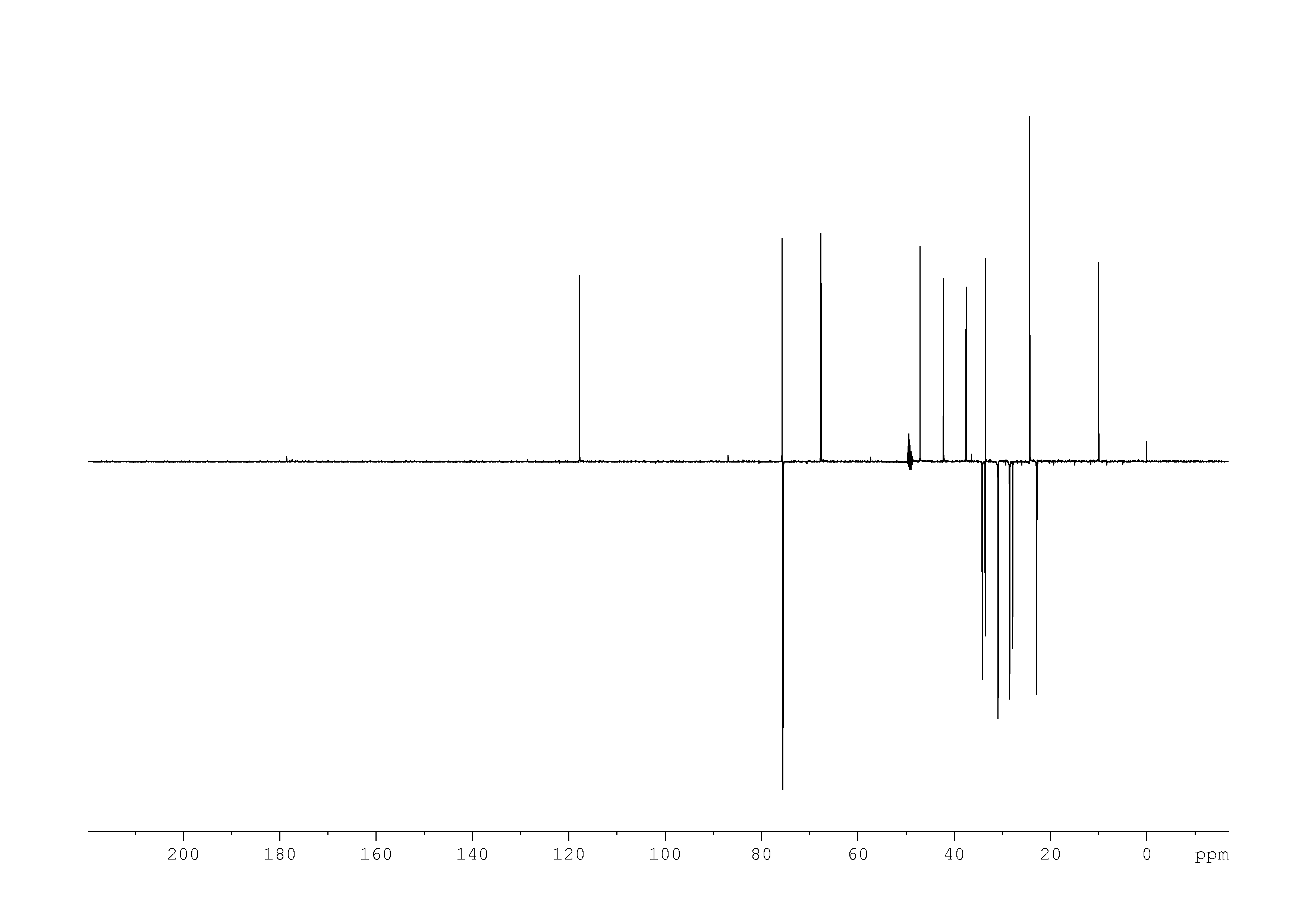
6: 2D [1H,13C]-HSQC
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #6: 2D [1H,13C]-HSQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000735/nmr/set01/spectra/1H_13C_HSQC.png)
7: 2D [1H,13C]-HMBC
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #7: 2D [1H,13C]-HMBC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000735/nmr/set01/spectra/1H_13C_HMBC.png)
8: 2D [1H,1H]-COSY
Sample: Saturated1 in methanol, ref: TMS
Conditions: temperature: 298K, pH: n/a
Spectrometer: Bruker DMX - 500MHz
![Spectrum for experiment #8: 2D [1H,1H]-COSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000735/nmr/set01/spectra/HH_COSY.png)
