NADP+ (C21H29N7O17P3+)
BMRB entry bmse000263
Entry DOI: doi:10.13018/BMSE000263
Data source: Madison Metabolomics Consortium - Qiu Cui, Ian Lewis, Mark E. Anderson, John L. Markley
NMR-STAR file:
bmse000263.strNMR-STAR
interactive viewerStructure file (mol/sdf):
bmse000263.molAll files for
bmse000263Time Domain Data:
bmse000263.zipSample and instrument details are given with the spectrum
Assigned chemical shifts
Set 1
Sample: saturatedmM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
| Atom ID | Author Nomenclature | Value | Ambiguity Code |
|---|---|---|---|
| C5 | C28 | 68.219 | 4 |
| C5 | C28 | 67.675 | 4 |
| C6 | C29 | 68.219 | 4 |
| C6 | C29 | 67.675 | 4 |
| C20 | C30 | 89.822 | 4 |
| C20 | C30 | 89.734 | 4 |
| C20 | C30 | 89.254 | 4 |
| C20 | C30 | 89.172 | 4 |
| C20 | C30 | 85.747 | 4 |
| C20 | C30 | 85.658 | 4 |
| C20 | C30 | 80.322 | 4 |
| C20 | C30 | 78.764 | 4 |
| C20 | C30 | 78.722 | 4 |
| C20 | C30 | 73.373 | 4 |
| C20 | C30 | 72.734 | 4 |
| C20 | C30 | 102.729 | 4 |
| C21 | C31 | 89.822 | 4 |
| C21 | C31 | 89.734 | 4 |
| C21 | C31 | 89.254 | 4 |
| C21 | C31 | 89.172 | 4 |
| C21 | C31 | 85.747 | 4 |
| C21 | C31 | 85.658 | 4 |
| C21 | C31 | 80.322 | 4 |
| C21 | C31 | 78.764 | 4 |
| C21 | C31 | 78.722 | 4 |
| C21 | C31 | 73.373 | 4 |
| C21 | C31 | 72.734 | 4 |
| C21 | C31 | 102.729 | 4 |
| C10 | C32 | 89.822 | 4 |
| C10 | C32 | 89.734 | 4 |
| C10 | C32 | 89.254 | 4 |
| C10 | C32 | 89.172 | 4 |
| C10 | C32 | 85.747 | 4 |
| C10 | C32 | 85.658 | 4 |
| C10 | C32 | 80.322 | 4 |
| C10 | C32 | 78.764 | 4 |
| C10 | C32 | 78.722 | 4 |
| C10 | C32 | 73.373 | 4 |
| C10 | C32 | 72.734 | 4 |
| C11 | C33 | 89.822 | 4 |
| C11 | C33 | 89.734 | 4 |
| C11 | C33 | 89.254 | 4 |
| C11 | C33 | 89.172 | 4 |
| C11 | C33 | 85.747 | 4 |
| C11 | C33 | 85.658 | 4 |
| C11 | C33 | 80.322 | 4 |
| C11 | C33 | 78.764 | 4 |
| C11 | C33 | 78.722 | 4 |
| C11 | C33 | 73.373 | 4 |
| C11 | C33 | 72.734 | 4 |
| C15 | C34 | 89.822 | 4 |
| C15 | C34 | 89.734 | 4 |
| C15 | C34 | 89.254 | 4 |
| C15 | C34 | 89.172 | 4 |
| C15 | C34 | 85.747 | 4 |
| C15 | C34 | 85.658 | 4 |
| C15 | C34 | 80.322 | 4 |
| C15 | C34 | 78.764 | 4 |
| C15 | C34 | 78.722 | 4 |
| C15 | C34 | 73.373 | 4 |
| C15 | C34 | 72.734 | 4 |
| C16 | C35 | 89.822 | 4 |
| C16 | C35 | 89.734 | 4 |
| C16 | C35 | 89.254 | 4 |
| C16 | C35 | 89.172 | 4 |
| C16 | C35 | 85.747 | 4 |
| C16 | C35 | 85.658 | 4 |
| C16 | C35 | 80.322 | 4 |
| C16 | C35 | 78.764 | 4 |
| C16 | C35 | 78.722 | 4 |
| C16 | C35 | 73.373 | 4 |
| C16 | C35 | 72.734 | 4 |
| C13 | C36 | 89.822 | 4 |
| C13 | C36 | 89.734 | 4 |
| C13 | C36 | 89.254 | 4 |
| C13 | C36 | 89.172 | 4 |
| C13 | C36 | 85.747 | 4 |
| C13 | C36 | 85.658 | 4 |
| C13 | C36 | 80.322 | 4 |
| C13 | C36 | 78.764 | 4 |
| C13 | C36 | 78.722 | 4 |
| C13 | C36 | 73.373 | 4 |
| C13 | C36 | 72.734 | 4 |
| C14 | C37 | 89.822 | 4 |
| C14 | C37 | 89.734 | 4 |
| C14 | C37 | 89.254 | 4 |
| C14 | C37 | 89.172 | 4 |
| C14 | C37 | 85.747 | 4 |
| C14 | C37 | 85.658 | 4 |
| C14 | C37 | 80.322 | 4 |
| C14 | C37 | 78.764 | 4 |
| C14 | C37 | 78.722 | 4 |
| C14 | C37 | 73.373 | 4 |
| C14 | C37 | 72.734 | 4 |
| C18 | C38 | 167.850 | 1 |
| C17 | C39 | 158.115 | 1 |
| C19 | C40 | 151.676 | 1 |
| C12 | C41 | 121.161 | 1 |
| C9 | C42 | 136.304 | 1 |
| C8 | C43 | 142.889 | 4 |
| C8 | C43 | 142.548 | 4 |
| C7 | C44 | 155.428 | 1 |
| C4 | C45 | 142.889 | 4 |
| C4 | C45 | 142.548 | 4 |
| C3 | C46 | 148.375 | 4 |
| C3 | C46 | 145.017 | 4 |
| C2 | C47 | 148.375 | 4 |
| C2 | C47 | 145.017 | 4 |
| C1 | C48 | 131.345 | 1 |
| H53 | H60 | 4.297 | 4 |
| H53 | H60 | 4.208 | 4 |
| H54 | H61 | 4.297 | 4 |
| H54 | H61 | 4.208 | 4 |
| H55 | H62 | 4.297 | 4 |
| H55 | H62 | 4.208 | 4 |
| H56 | H63 | 4.297 | 4 |
| H56 | H63 | 4.208 | 4 |
| H65 | H64 | 6.019 | 4 |
| H66 | H65 | 4.958 | 4 |
| H66 | H65 | 4.603 | 4 |
| H66 | H65 | 4.468 | 4 |
| H66 | H65 | 4.380 | 4 |
| H66 | H65 | 6.087 | 4 |
| H66 | H65 | 6.019 | 4 |
| H59 | H66 | 4.958 | 4 |
| H59 | H66 | 4.603 | 4 |
| H59 | H66 | 4.468 | 4 |
| H59 | H66 | 4.380 | 4 |
| H60 | H67 | 4.958 | 4 |
| H60 | H67 | 4.603 | 4 |
| H60 | H67 | 4.468 | 4 |
| H60 | H67 | 4.380 | 4 |
| H63 | H68 | 4.958 | 4 |
| H63 | H68 | 4.603 | 4 |
| H63 | H68 | 4.468 | 4 |
| H63 | H68 | 4.380 | 4 |
| H64 | H69 | 4.958 | 4 |
| H64 | H69 | 4.603 | 4 |
| H64 | H69 | 4.468 | 4 |
| H64 | H69 | 4.380 | 4 |
| H61 | H70 | 4.958 | 4 |
| H61 | H70 | 4.603 | 4 |
| H61 | H70 | 4.468 | 4 |
| H61 | H70 | 4.380 | 4 |
| H62 | H71 | 4.958 | 4 |
| H62 | H71 | 4.603 | 4 |
| H62 | H71 | 4.468 | 4 |
| H62 | H71 | 4.380 | 4 |
| H58 | H72 | 9.281 | 4 |
| H58 | H72 | 8.406 | 4 |
| H57 | H73 | 8.125 | 1 |
| H52 | H74 | 9.281 | 4 |
| H52 | H74 | 8.406 | 4 |
| H51 | H75 | 9.093 | 4 |
| H51 | H75 | 8.802 | 4 |
| H50 | H76 | 9.093 | 4 |
| H50 | H76 | 8.802 | 4 |
| H49 | H77 | 8.171 | 1 |
NMR experiments
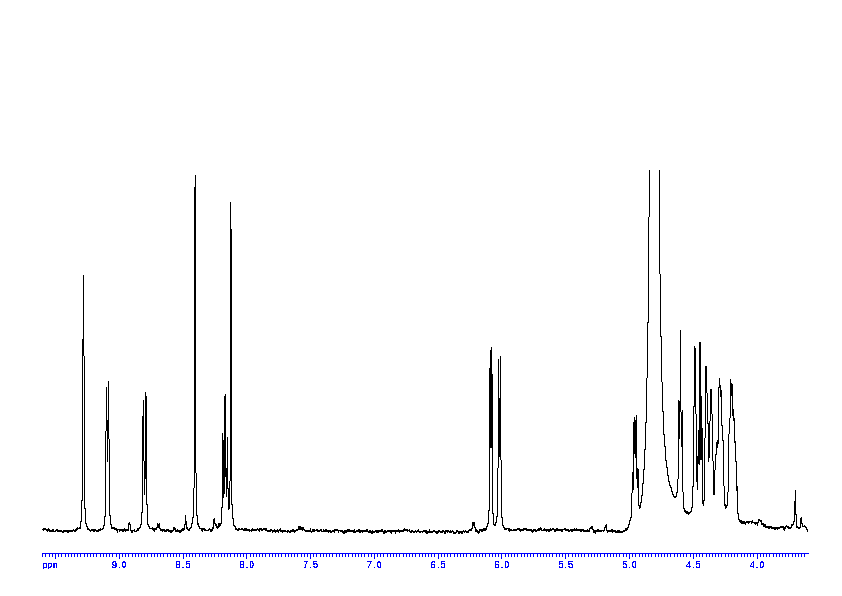
1: 1D 1H
Sample: saturatedmM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
2: 2D [1H,1H]-TOCSY
Sample: saturatedmM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
![Spectrum for experiment #2: 2D [1H,1H]-TOCSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000263/nmr/set01/spectra/HH_TOCSY.png)
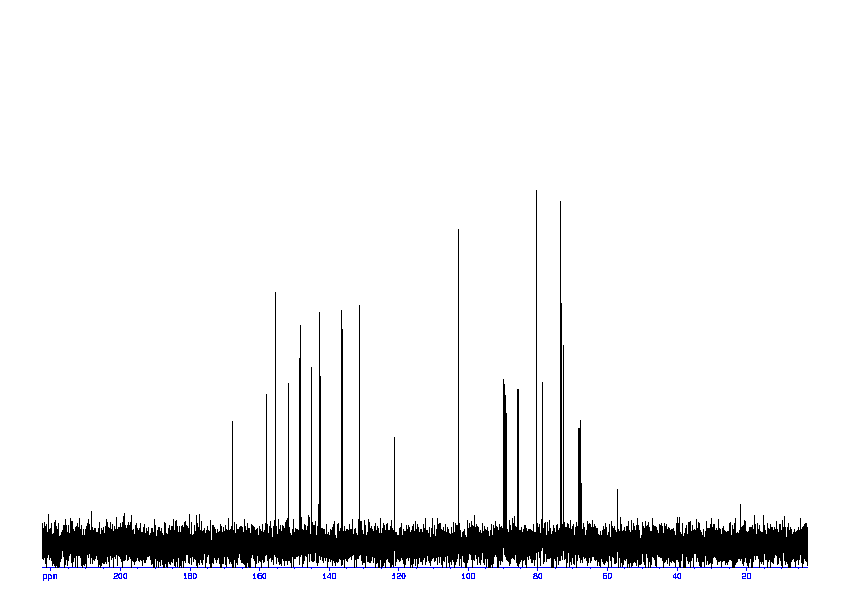
3: 1D 13C
Sample: saturatedmM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
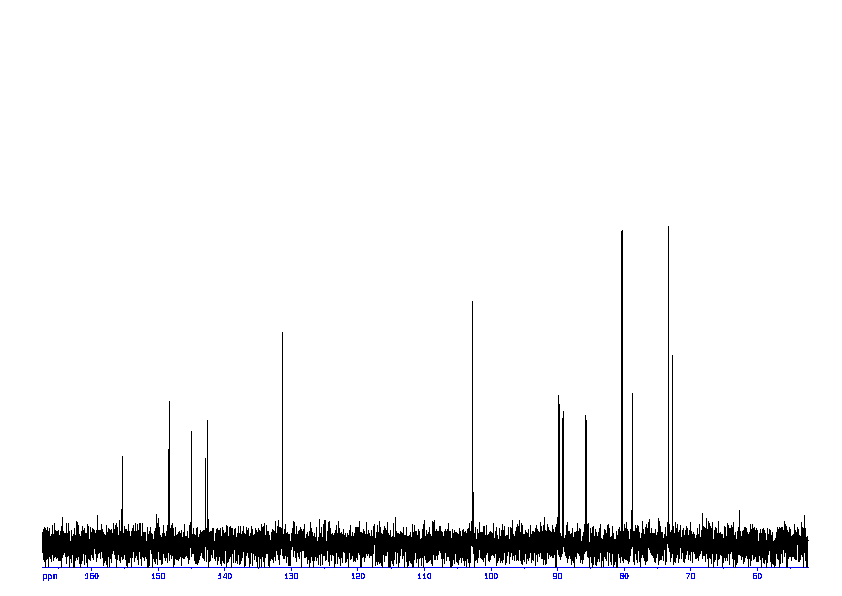
4: 1D DEPT90
Sample: saturatedmM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
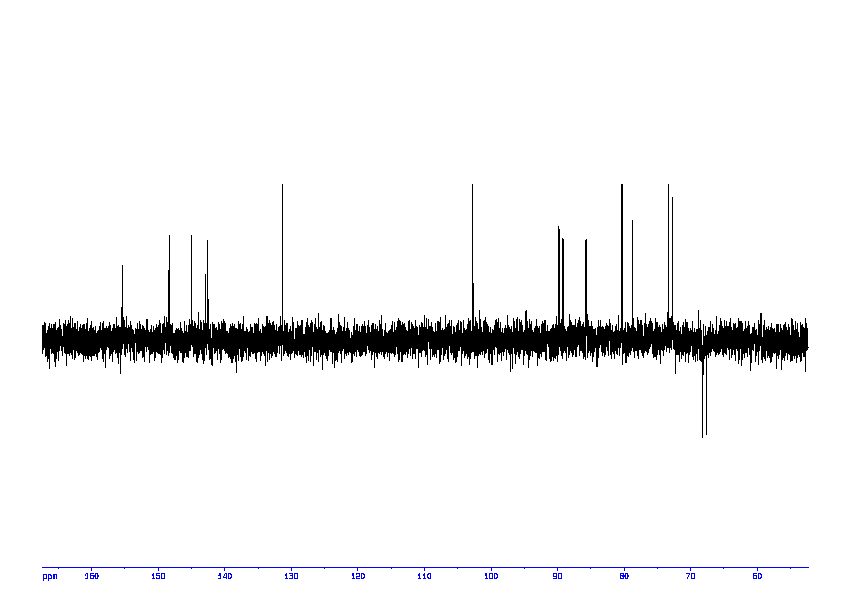
5: 1D DEPT135
Sample: saturatedmM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
6: 2D [1H,13C]-HSQC
Sample: saturatedmM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
![Spectrum for experiment #6: 2D [1H,13C]-HSQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000263/nmr/set01/spectra/1H_13C_HSQC.png)
