FAD (C27H33N9O15P2)
BMRB entry bmse000226
Entry DOI: doi:10.13018/BMSE000226
Data source: Madison Metabolomics Consortium - Qiu Cui, Ian Lewis, Mark E. Anderson, John L. Markley
NMR-STAR file:
bmse000226.strNMR-STAR
interactive viewerStructure file (mol/sdf):
bmse000226.molAll files for
bmse000226Time Domain Data:
bmse000226.zipSample and instrument details are given with the spectrum
Assigned chemical shifts
Set 1
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
| Atom ID | Author Nomenclature | Value | Ambiguity Code |
|---|---|---|---|
| C1 | C1 | 23.450 | 4 |
| C1 | C1 | 21.306 | 4 |
| C2 | C2 | 23.450 | 4 |
| C2 | C2 | 21.306 | 4 |
| C3 | C3 | 119.139 | 1 |
| C4 | C4 | 133.115 | 1 |
| C8 | C5 | 155.040 | 1 |
| C9 | C6 | 141.803 | 1 |
| C7 | C7 | 70.552 | 4 |
| C7 | C7 | 67.838 | 4 |
| C5 | C8 | 49.830 | 1 |
| C6 | C9 | 70.552 | 4 |
| C6 | C9 | 67.838 | 4 |
| C10 | C10 | 164.069 | 4 |
| C10 | C10 | 160.464 | 4 |
| C10 | C10 | 157.401 | 4 |
| C10 | C10 | 153.202 | 4 |
| C10 | C10 | 150.751 | 4 |
| C10 | C10 | 137.302 | 4 |
| C10 | C10 | 136.720 | 4 |
| C10 | C10 | 134.113 | 4 |
| C10 | C10 | 120.401 | 4 |
| C11 | C11 | 164.069 | 4 |
| C11 | C11 | 160.464 | 4 |
| C11 | C11 | 157.401 | 4 |
| C11 | C11 | 153.202 | 4 |
| C11 | C11 | 150.751 | 4 |
| C11 | C11 | 137.302 | 4 |
| C11 | C11 | 136.720 | 4 |
| C11 | C11 | 134.113 | 4 |
| C11 | C11 | 120.401 | 4 |
| C12 | C12 | 164.069 | 4 |
| C12 | C12 | 160.464 | 4 |
| C12 | C12 | 157.401 | 4 |
| C12 | C12 | 153.202 | 4 |
| C12 | C12 | 150.751 | 4 |
| C12 | C12 | 137.302 | 4 |
| C12 | C12 | 136.720 | 4 |
| C12 | C12 | 134.113 | 4 |
| C12 | C12 | 120.401 | 4 |
| C13 | C13 | 164.069 | 4 |
| C13 | C13 | 160.464 | 4 |
| C13 | C13 | 157.401 | 4 |
| C13 | C13 | 153.202 | 4 |
| C13 | C13 | 150.751 | 4 |
| C13 | C13 | 137.302 | 4 |
| C13 | C13 | 136.720 | 4 |
| C13 | C13 | 134.113 | 4 |
| C13 | C13 | 120.401 | 4 |
| C17 | C14 | 164.069 | 4 |
| C17 | C14 | 160.464 | 4 |
| C17 | C14 | 157.401 | 4 |
| C17 | C14 | 153.202 | 4 |
| C17 | C14 | 150.751 | 4 |
| C17 | C14 | 137.302 | 4 |
| C17 | C14 | 136.720 | 4 |
| C17 | C14 | 134.113 | 4 |
| C17 | C14 | 120.401 | 4 |
| C18 | C15 | 164.069 | 4 |
| C18 | C15 | 160.464 | 4 |
| C18 | C15 | 157.401 | 4 |
| C18 | C15 | 153.202 | 4 |
| C18 | C15 | 150.751 | 4 |
| C18 | C15 | 137.302 | 4 |
| C18 | C15 | 136.720 | 4 |
| C18 | C15 | 134.113 | 4 |
| C18 | C15 | 120.401 | 4 |
| C22 | C16 | 164.069 | 4 |
| C22 | C16 | 160.464 | 4 |
| C22 | C16 | 157.401 | 4 |
| C22 | C16 | 153.202 | 4 |
| C22 | C16 | 150.751 | 4 |
| C22 | C16 | 137.302 | 4 |
| C22 | C16 | 136.720 | 4 |
| C22 | C16 | 134.113 | 4 |
| C22 | C16 | 120.401 | 4 |
| C25 | C17 | 206.988 | 4 |
| C23 | C18 | 164.069 | 4 |
| C23 | C18 | 160.464 | 4 |
| C23 | C18 | 157.401 | 4 |
| C23 | C18 | 153.202 | 4 |
| C23 | C18 | 150.751 | 4 |
| C23 | C18 | 137.302 | 4 |
| C23 | C18 | 136.720 | 4 |
| C23 | C18 | 134.113 | 4 |
| C23 | C18 | 120.401 | 4 |
| C24 | C19 | 164.069 | 4 |
| C24 | C19 | 160.464 | 4 |
| C24 | C19 | 157.401 | 4 |
| C24 | C19 | 153.202 | 4 |
| C24 | C19 | 150.751 | 4 |
| C24 | C19 | 137.302 | 4 |
| C24 | C19 | 136.720 | 4 |
| C24 | C19 | 134.113 | 4 |
| C24 | C19 | 120.401 | 4 |
| C27 | C20 | 206.988 | 4 |
| C16 | C21 | 86.255 | 1 |
| C20 | C22 | 77.590 | 4 |
| C20 | C22 | 75.190 | 4 |
| C20 | C22 | 73.887 | 4 |
| C20 | C22 | 72.674 | 4 |
| C20 | C22 | 72.020 | 4 |
| C21 | C23 | 77.590 | 4 |
| C21 | C23 | 75.190 | 4 |
| C21 | C23 | 73.887 | 4 |
| C21 | C23 | 72.674 | 4 |
| C21 | C23 | 72.020 | 4 |
| C26 | C24 | 89.829 | 1 |
| C14 | C25 | 77.590 | 4 |
| C14 | C25 | 75.190 | 4 |
| C14 | C25 | 73.887 | 4 |
| C14 | C25 | 72.674 | 4 |
| C14 | C25 | 72.020 | 4 |
| C15 | C26 | 77.590 | 4 |
| C15 | C26 | 75.190 | 4 |
| C15 | C26 | 73.887 | 4 |
| C15 | C26 | 72.674 | 4 |
| C15 | C26 | 72.020 | 4 |
| C19 | C27 | 77.590 | 4 |
| C19 | C27 | 75.190 | 4 |
| C19 | C27 | 73.887 | 4 |
| C19 | C27 | 72.674 | 4 |
| C19 | C27 | 72.020 | 4 |
| H56 | H54 | 2.396 | 4 |
| H56 | H54 | 2.333 | 4 |
| H55 | H55 | 2.396 | 4 |
| H55 | H55 | 2.333 | 4 |
| H54 | H56 | 2.396 | 4 |
| H54 | H56 | 2.333 | 4 |
| H58 | H57 | 2.396 | 4 |
| H58 | H57 | 2.333 | 4 |
| H59 | H58 | 2.396 | 4 |
| H59 | H58 | 2.333 | 4 |
| H57 | H59 | 2.396 | 4 |
| H57 | H59 | 2.333 | 4 |
| H60 | H60 | 7.541 | 1 |
| H61 | H61 | 7.619 | 1 |
| H68 | H62 | 7.847 | 1 |
| H69 | H63 | 8.307 | 1 |
| H66 | H64 | 4.332 | 4 |
| H66 | H64 | 4.053 | 4 |
| H67 | H65 | 4.332 | 4 |
| H67 | H65 | 4.053 | 4 |
| H62 | H66 | 4.332 | 4 |
| H62 | H66 | 4.053 | 4 |
| H63 | H67 | 4.332 | 4 |
| H63 | H67 | 4.053 | 4 |
| H64 | H68 | 4.332 | 4 |
| H64 | H68 | 4.053 | 4 |
| H65 | H69 | 4.332 | 4 |
| H65 | H69 | 4.053 | 4 |
| H72 | H70 | 4.332 | 4 |
| H72 | H70 | 4.053 | 4 |
| H74 | H71 | 4.510 | 4 |
| H74 | H71 | 4.473 | 4 |
| H74 | H71 | 3.895 | 4 |
| H74 | H71 | 4.053 | 4 |
| H75 | H72 | 4.510 | 4 |
| H75 | H72 | 4.473 | 4 |
| H75 | H72 | 3.895 | 4 |
| H75 | H72 | 4.053 | 4 |
| H76 | H73 | 5.829 | 1 |
| H70 | H74 | 4.510 | 4 |
| H70 | H74 | 4.473 | 4 |
| H70 | H74 | 3.895 | 4 |
| H70 | H74 | 4.332 | 4 |
| H70 | H74 | 4.053 | 4 |
| H71 | H75 | 4.510 | 4 |
| H71 | H75 | 4.473 | 4 |
| H71 | H75 | 3.895 | 4 |
| H71 | H75 | 4.332 | 4 |
| H71 | H75 | 4.053 | 4 |
| H73 | H76 | 4.510 | 4 |
| H73 | H76 | 4.473 | 4 |
| H73 | H76 | 3.895 | 4 |
| H73 | H76 | 4.332 | 4 |
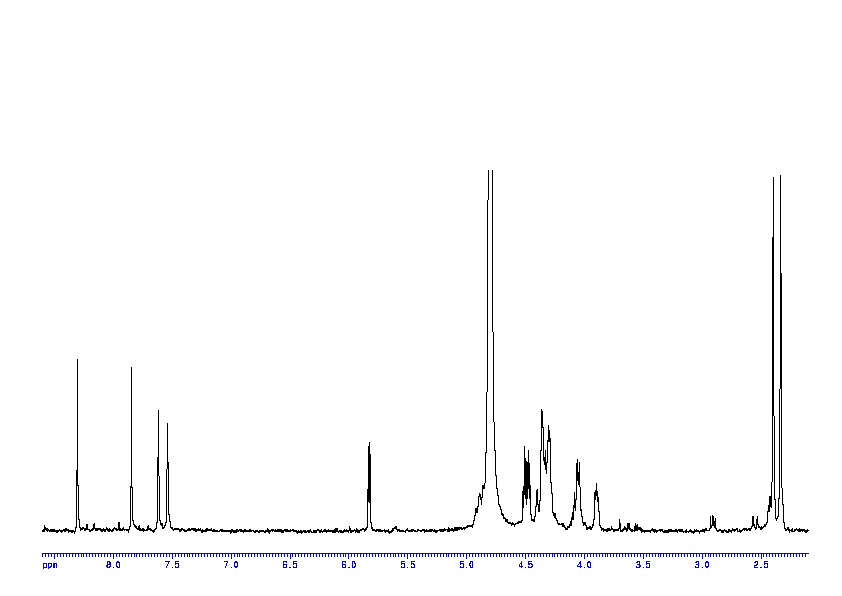
NMR experiments
1: 1D 1H
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
2: 2D [1H,1H]-TOCSY
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
![Spectrum for experiment #2: 2D [1H,1H]-TOCSY](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000226/nmr/set01/spectra/HH_TOCSY.png)
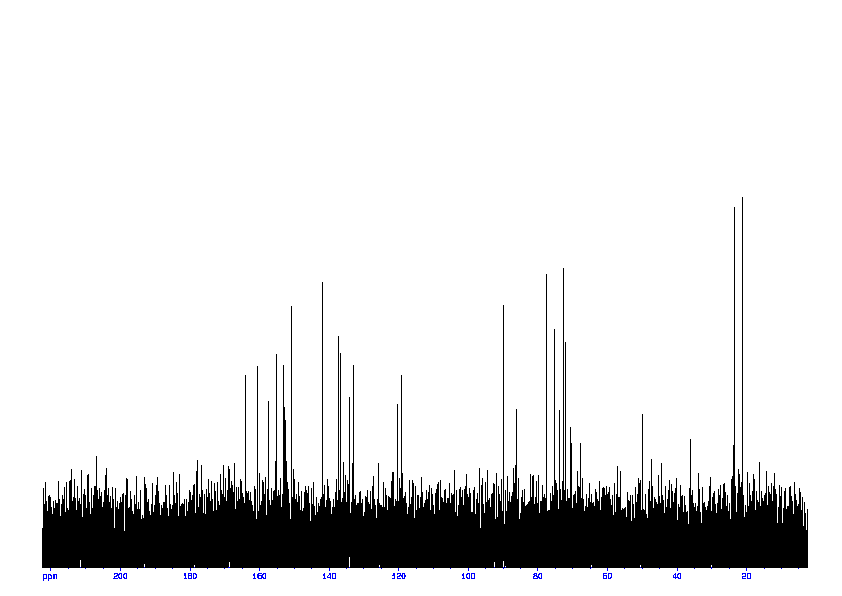
3: 1D 13C
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
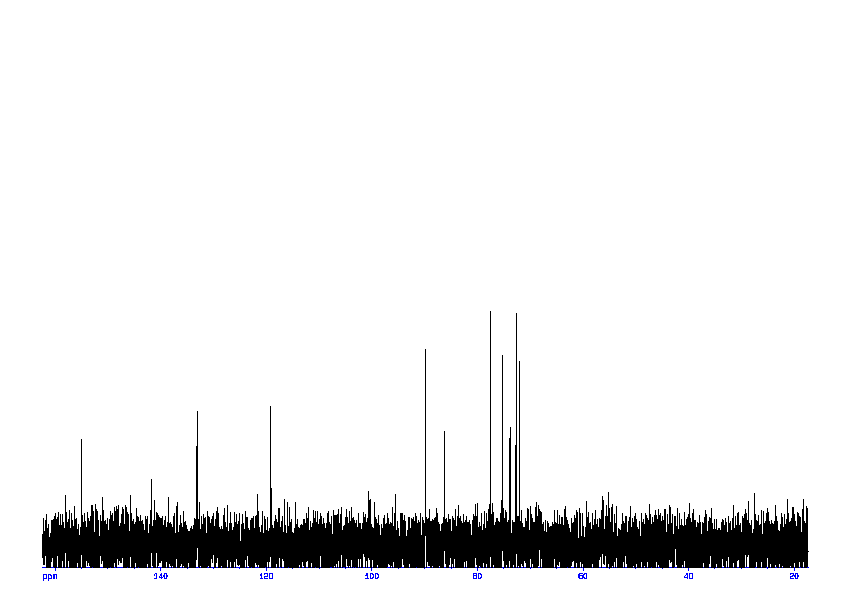
4: 1D DEPT90
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
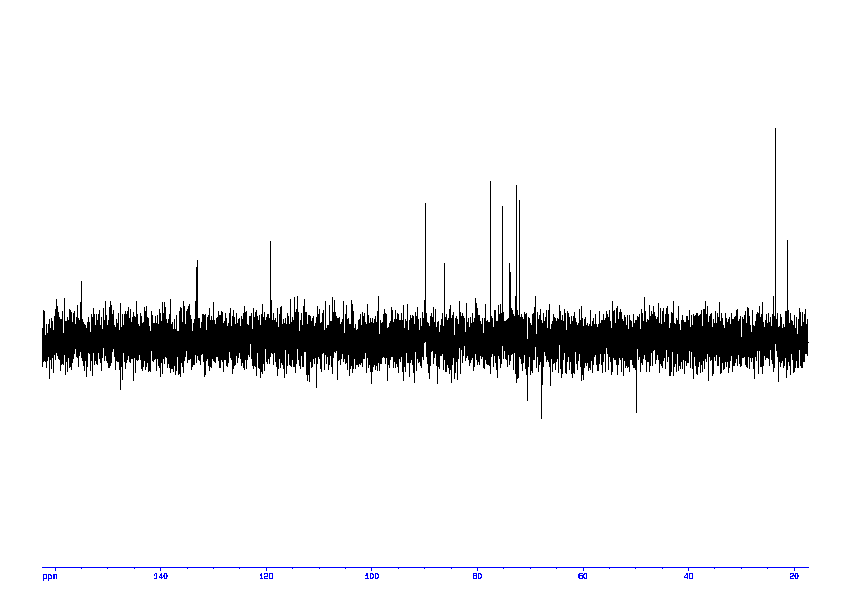
5: 1D DEPT135
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
6: 2D [1H,13C]-HSQC
Sample: 100mM in D2O, ref: DSS
Conditions: temperature: 298K, pH: 7.4
Spectrometer: Bruker DMX - 400MHz
![Spectrum for experiment #6: 2D [1H,13C]-HSQC](/ftp/pub/bmrb/metabolomics/entry_directories/bmse000226/nmr/set01/spectra/1H_13C_HSQC.png)
